In recent news, the European Commission passed an extension (EU Regulation 2023/607) to the transition deadline for Legacy Medical Devices to be placed on the EU market. Here is a quick summary of recently posted information about the reasoning behind this decision and how it affects medical device manufacturers.
Surveys
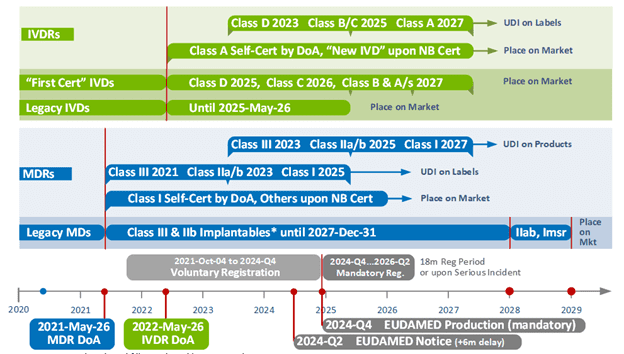
Currently Legacy Medical Devices, i.e., devices with a valid Medical Device Directive (MDD) or an Active Implantable Medical Device Directive (AIMDD) certificate and without any significant design changes, are able to be placed on the market under Medical Device Regulation (MDR) Article 120(3) until May 26, 2024. Two surveys conducted in 2022 confirmed the low number of designated Notified Bodies (now 36) are not expected to complete the necessary MDR assessments by the May 26, 2024 deadline, resulting in a shortage of medical devices on the EU market.
In response, on March 20, 2023, the European Commission (EC) and the European Council agreed to an Amendment to extend the transition period for Legacy Medical Devices to be placed on the market. That MDR amendment is to define the Legacy Medical Device transition extension.
Currently, legacy Medical Devices, i.e., devices with a valid Medical Device Directive (MDD) or an Active Implantable Medical Device Directive (AIMDD) certificate, and without any significant design changes, are able to be placed on the market under MDR Article 120(3) until May 26, 2024.
Amendment:
The amendment includes the following provisions:
- Extend the Legacy Device Class III and IIb Implantables transition period end date to place products on the market to December 31, 2027 [extended 3 years, 7 months]
- Extend the Legacy Device Class IIb non-Implantables, IIa, Imsr*, and up-classified “1st certificate” devices to December 31, 2028 transition period end date to place products on the market to December 31, 2028 (extended 4 years, 7 months)
- Extend the validity of Legacy MDD and AIMDD certificates [MDR Article 120(2)]
- Extend QMS certification deadline to 2026-May-26 for Class III custom-made implantable devices
- Remove the one-year “Sell-off” period for Legacy MD (and IVD) devices to flow through the supply chain.
Limit the extension only to Legacy Devices that:
- Do not present any unacceptable risk to health and safety
- Have not undergone significant changes in design or intended purpose
- For which the manufacturers have already undertaken the necessary steps to launch the certification process under the MDR
* (NB Conformity Assessment required, i.e., Class I Measuring Function, Sterile, and Reusable Surgical Instrument).
Updated EU MDR/IVDR Timeline
For More Information on Legacy Devices Watch this short video:
FYI
IMPORTANT - There is no mention in the amendment to change the mandatory UDI/Device Registration period to EUDAMED. As provided by the MDR, this period is triggered by the EC Notice that “EUDAMED is fully functional.” The mandatory EU UDI/Device Registration period is currently expected to be from Q4 2024 to Q2 2026.
This amendment only addresses Legacy Medical Devices. Legacy In-Vitro Diagnostic devices were previously granted a 1-year transition extension and extended dates for up-classified IVDs. Although some have indicated the low IVDR NB capacity and slow migration to IVDRs may need to be re-evaluated.
As more information comes in, we will continue to report on this evolving process. For additional information about EUDAMED and other Medical Device resources, please visit our Knowledge Center.
Questions about health authorities and Unique Device Identification (UDI)? We monitor health authorities around the globe for the latest requirements and exceptions. If you have UDI questions, we can help. Email us: [email protected] or call +1-215-557-3010
