SaaS, Premium SaaS & GDSN
SingleSource™ for Medical Devices
Global UDI Submission SaaS Solution
Manage UDI data records by direct interface to a cloud-based solution
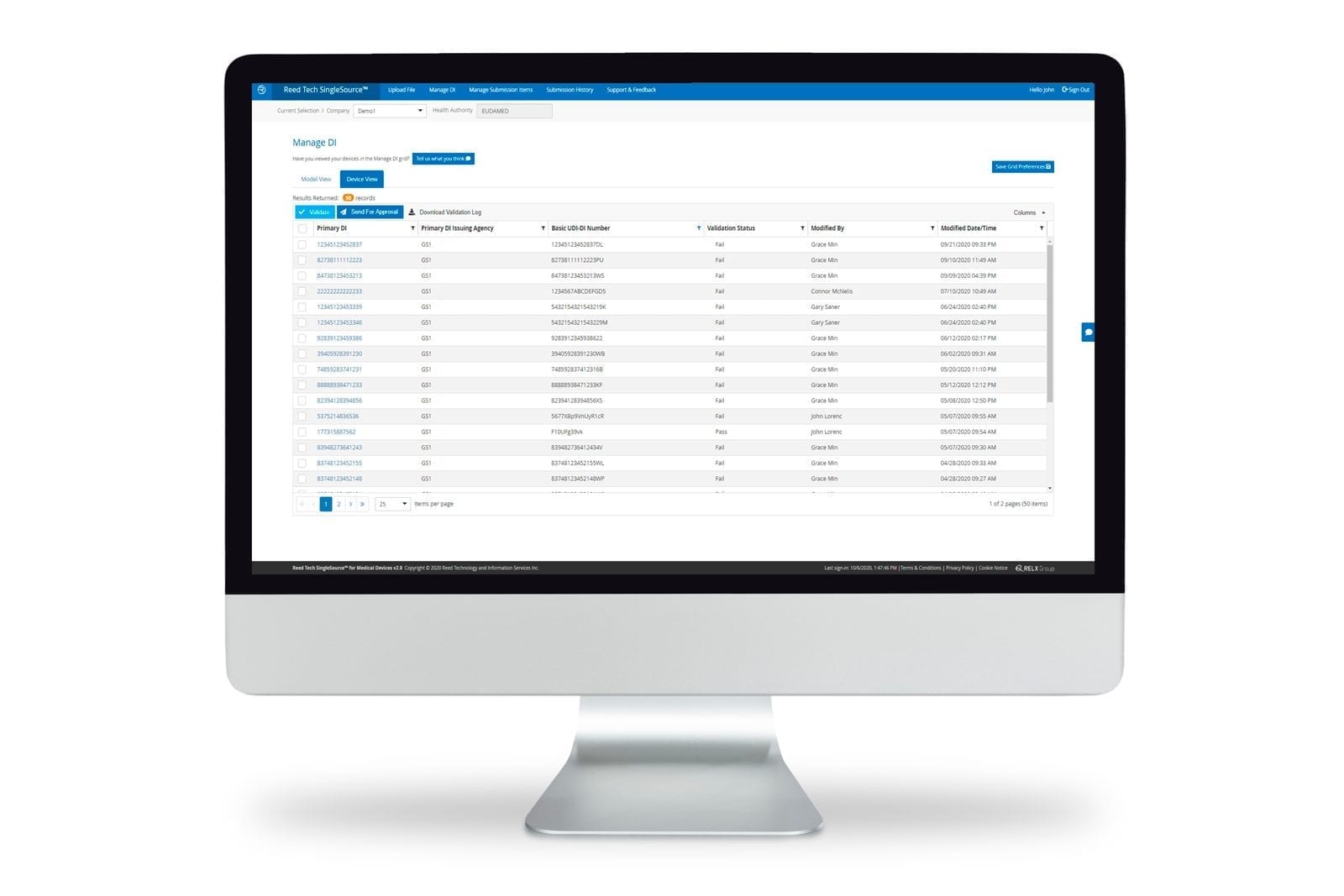
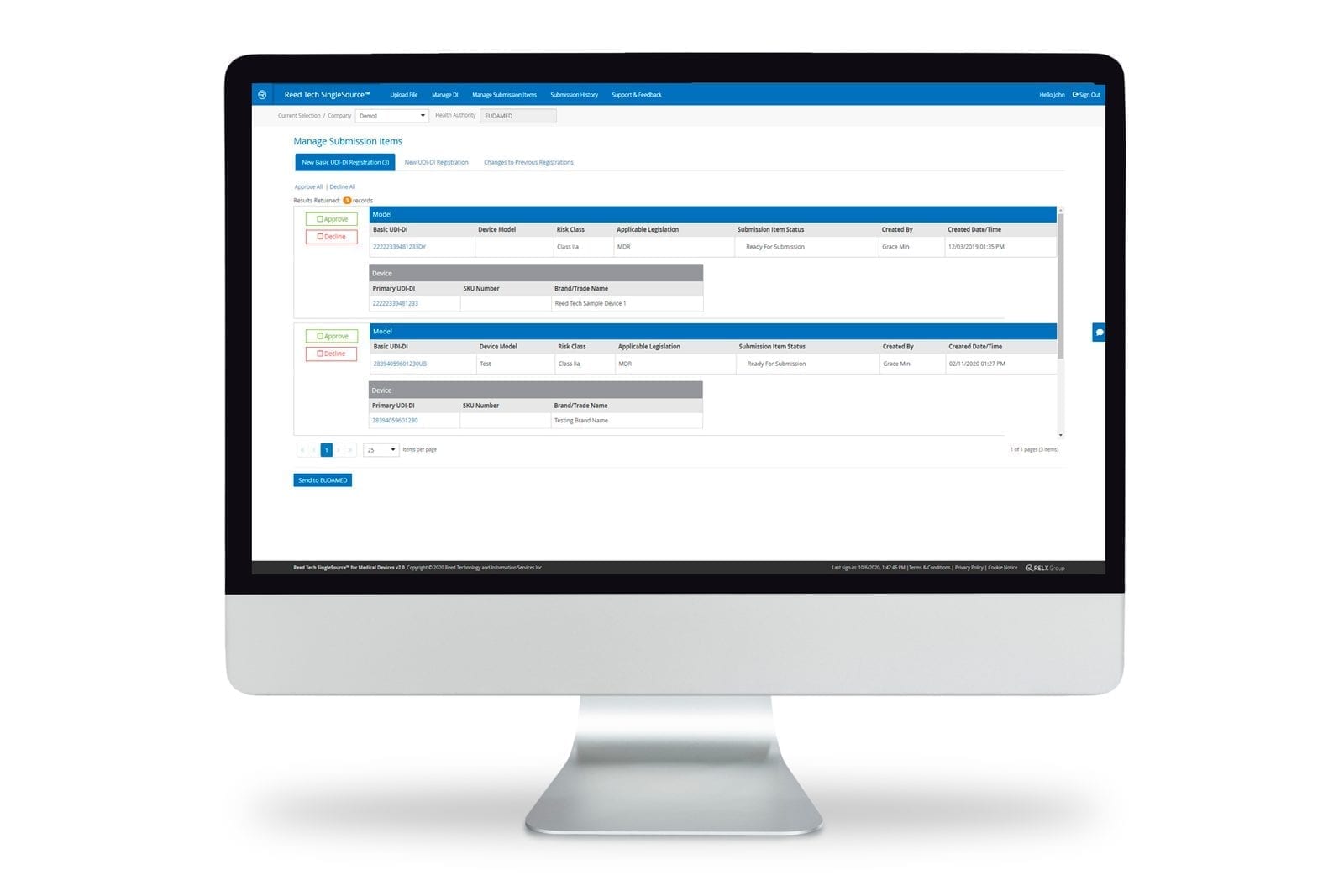
Medical device labelers can securely manage UDI record submission and product data lifecycle management directly via the SingleSource™ user interface. SingleSource™ supports FDA GUDID submissions and future UDI regulatory requirements of Global Health Authorities. By managing product data records in the cloud, regulatory and compliance staff can perform all the necessary UDI functions throughout lifecycle changes:
- Importing UDI data files
- Extracting records, transforming and loading data
- Validating the UDI data value against FDA and Reed Tech business rules
- Submitting SPL-UDI messages to FDA
- Processing and storing of FDA Acknowledgement (ACK) messages
SingleSource™ UDI Premium SaaS Solution
Guided, full-service approach to UDI record submission and management
For device labelers with limited staff resources to dedicate to UDI record management, the SingleSource™ UDI Premium SaaS Solution provides guided-support UDI submission. The experienced staff at Reed Tech will help:
- Collect and validate UDI data
- Build UDI records in HL7 Structured Product Labeling (SPL) format
- Submit records electronically to FDA GUDID
- Perform ongoing record lifecycle management
- FDA GUDID and future Health Authorities around the globe
- Schedule 1:1 and group training sessions
Class I Basic Account
Help is here for the FDA Class I UDI Submission Deadline
- Simple step-by-step guidance for registration
- Validation and acknowledgment
- Submission meets all technical components of CFR Part 11 requirements
- Ideal for 25 records or less to submit
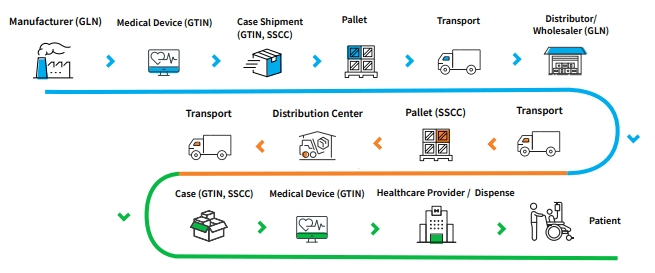
Global Data Synchronization Network (GDSN) Connection
A full-service approach to syndicate product data
GDSN syndication of product data allows device organizations to meet the requirements of governmental authorities, including UK NHS eProcurement and buyers, Group Purchasing Organizations (GPOs) and influential hospital networks.
- Share medical device product data with trading partners through the Global Data Synchronization Network (GDSN)
- Fulfill United Kingdom National Health Service eProcurement requirementsProvide product data to customers, including Group Purchasing Organizations (GPOs) and hospital networks