Medical Device Unique Device Identification (UDI)
Experienced UDI Data Submission to Global Health Authorities
Easily maintain a source data set and provide accurate product data to regulatory authorities and customers
Reed Tech is a leading FDA supplier of Unique Device Identification (UDI) information, submitting close to one-quarter of all NLM Access GUDID UDI records annually, representing about 34% of electronic SPL submissions to GUDID.
With SingleSource™ for Medical Devices, UDI data is managed throughout the product lifecycle in a compliant SaaS environment and includes scalability for additional volume and global health authorities. Reed Tech has active UDI channels for US FDA, China NMPA, South Korea MFDS and staging for EU EUDAMED and Australia UDID.
Learn about regulations and requirements for global health authorities. Click here to access the ‘Understanding UDI’ series.
Global UDI Summary by Region
Below is a high-level summary of UDI-related activities by region. It includes UDI plans and timelines for various regulatory authorities and commercial trading partners. As the number of global entities requiring medical device UDI database information grows, Reed Tech will add selectable channels for data submission to regulatory bodies and data syndication to commercial trading partners. Strategic product data management supported by a deep understanding of medical device regulatory and user UDI data requirements will ultimately contribute to improved outcomes for both healthcare providers and patients.
GLOBAL UDI REQUIREMENTS BY REGION
UDI Global
Learn about regulations and requirements for global health authorities. Click here to access the ‘Understanding UDI’ series.
What is UDI?
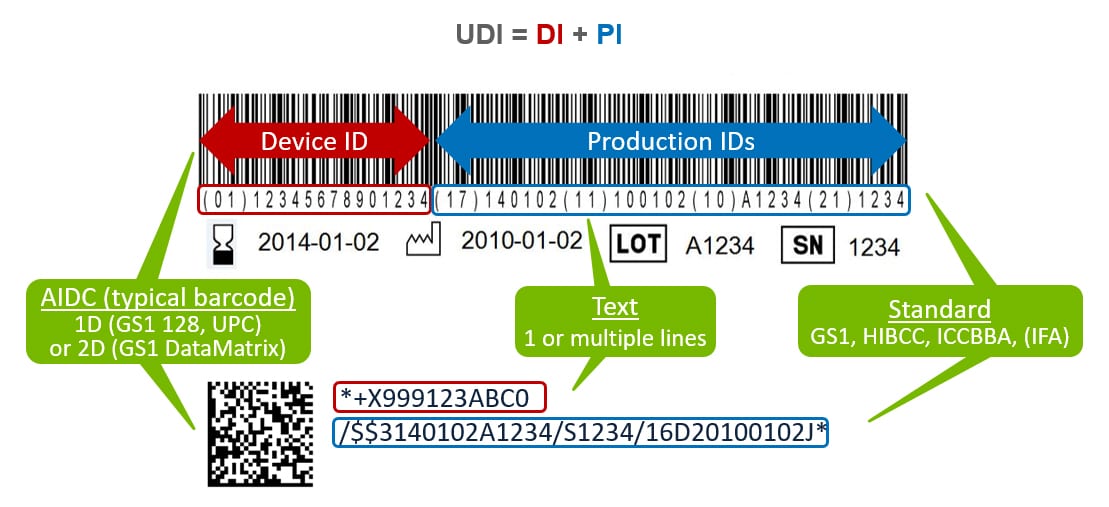
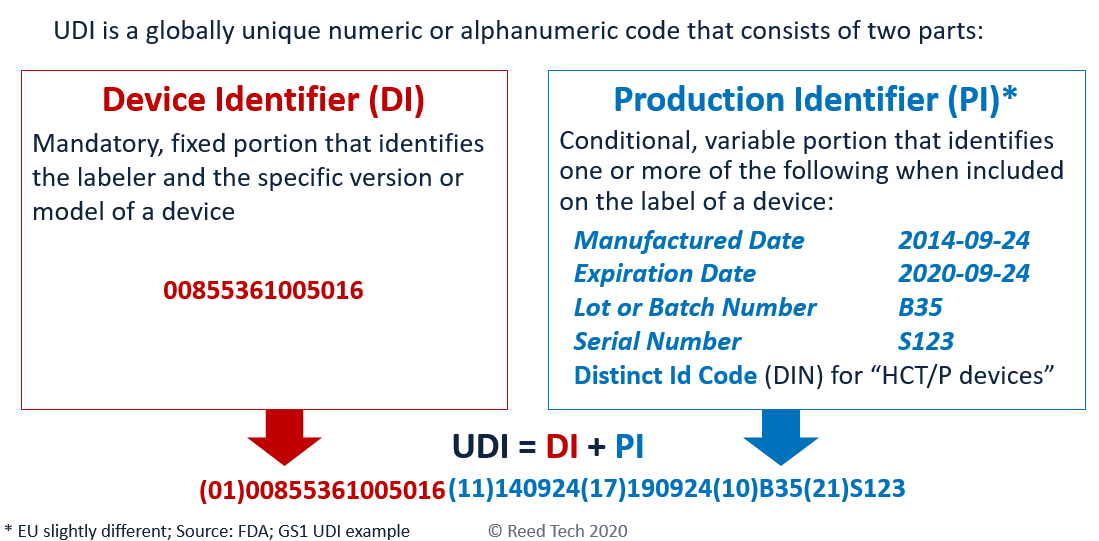
Unique Device Identification, or UDI is a regulatory requirement first enacted by the US FDA, and now adapted by regulatory agencies around the world. UDI enhances patient safety by identifying each product with a static device identifier denoting the device labeler and the specific model or version of a device and the dynamic product identifier which identifies the expiration date, serial number, manufactured date or the lot/batch number of the device. While the UDI is created through the guidelines of an approved issuing agency, the medical device manufacturer is responsible for performing a submission of the identifier along with several product data attributes. If all of this sounds confusing, Reed Tech is here to help.
Speak to a Subject-Matter Expert
SingleSource™ for Medical Devices was built to meet the product data management needs of Medical Device manufacturers. See what it can do for you.
Sign up for a demo today.