SingleSource™ for Drug Products helps regulatory professionals manage drug product meta-data with an intuitive interface, filters, and prompts. With new enhancements soon to be released, SingleSource™ for Drug Products is even more intuitive and user-friendly.
Reed Tech has recently revamped and improved SingleSource™ for Drug Products with the customer experience in mind. To help users save time and increase productivity, SingleSource now incorporates clean, intuitive forms and automatic defaults in a smarter system that anticipate user needs. The thorough data ingestion process ensures there is no starting from scratch. Throughout the forms, consistency is key allowing for a smooth, easily navigated process.
Let’s take a closer look at some of the specific changes:
New User Authentication
- Users are now fully in control of how they log in
- Reset passwords now at the user level – eliminating any wait time
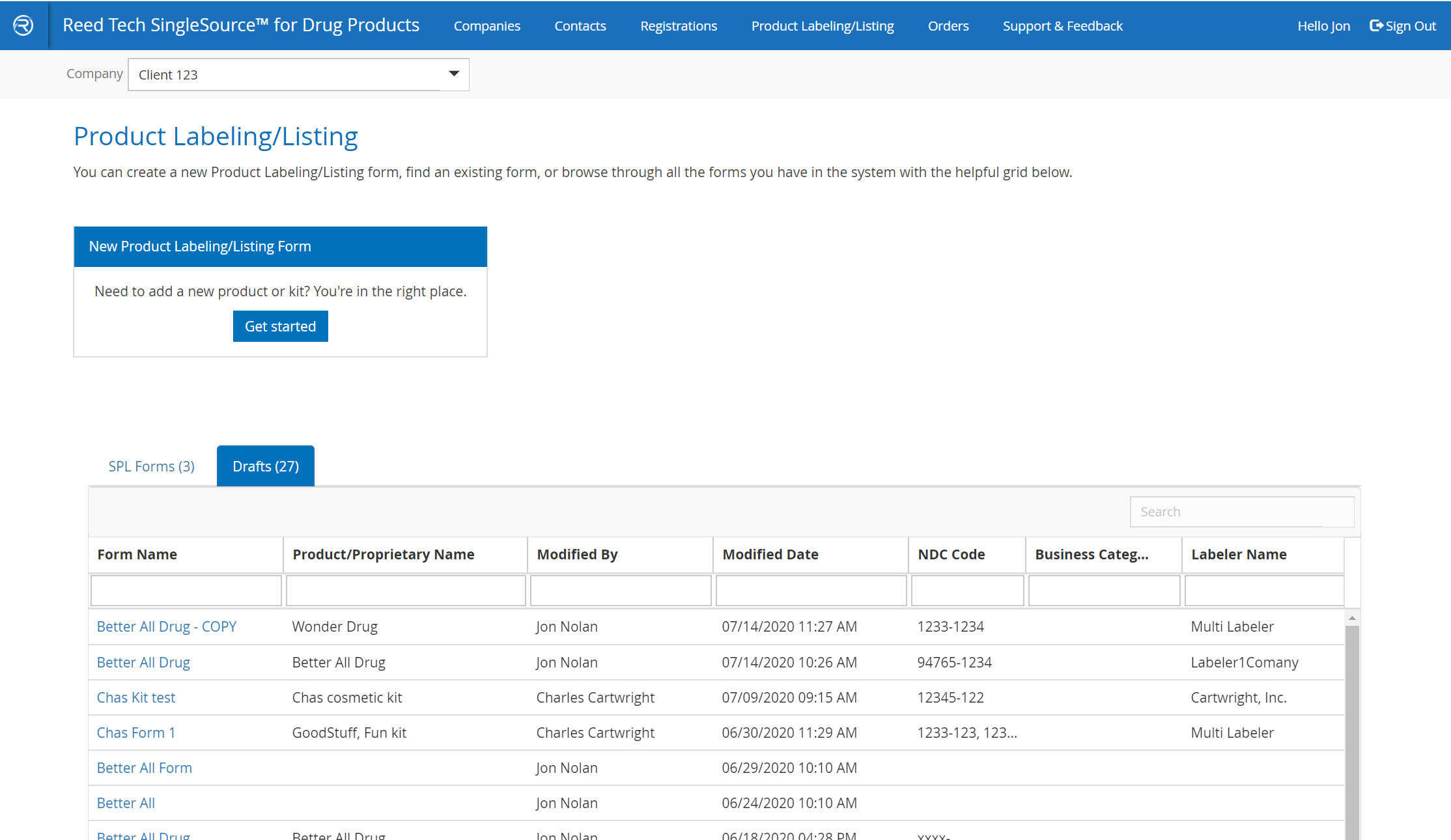
Order Tracker page
- All processed orders appear here
- Retrieve deliveries and download, accept, resubmit, or cancel orders
- Draft orders are now visible to the side
- Order activity is arranged in reverse chronological order
- Orders can be sorted or filtered, as well as a combined search feature
Add an Order Process
- Now 3 steps: select the order type (full conversion, partial conversion, or ESG FDA submission,) service type, and delivery priority
- Better traceability with customized order names
- Source documentation can now be mass uploaded with drag and drop functionality
- Optional instruction boxes have been added as well
- Save as a draft order functionality during any of the three steps
Labeling and Listing Page Enhancements
- Totally redesigned to provide a familiar look and feel
- Now supports copy and paste functionality to assist with lengthy fields
- NDC codes added to find products more quickly
- Company name added for clarity
- FDA Substance Registration System direct link added
- Only active versions are visible in the grid (removed draft versions)
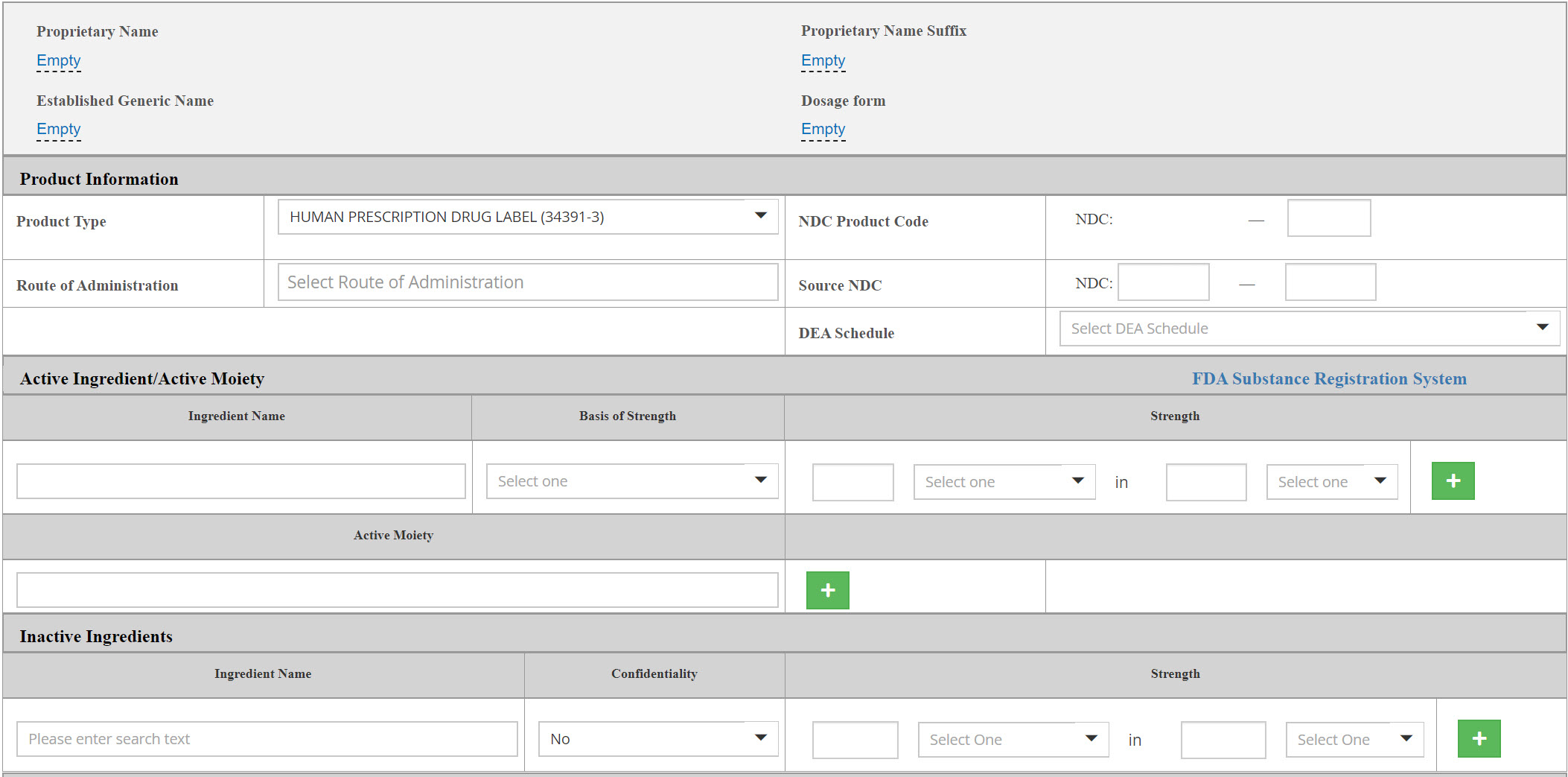
New easy-to-use Labeling /Listing Form
- Audit trails to previous versions are available and can be re-used
- Newly added expandable toolbar featuring Set ID value
- Automatic assignment of all business operations that are associated with the selected company and establishment
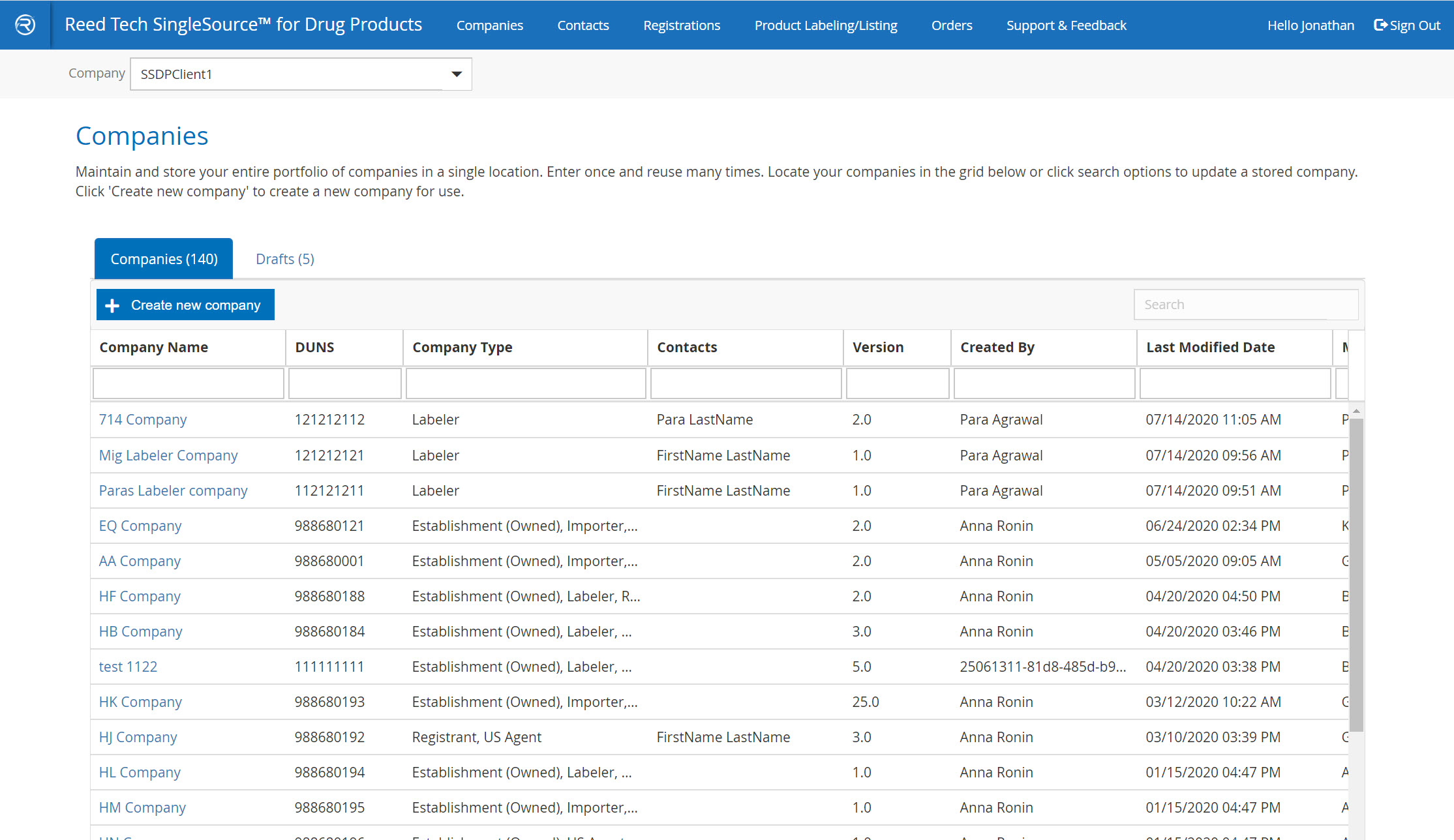
Easy-to-search, transparent grids
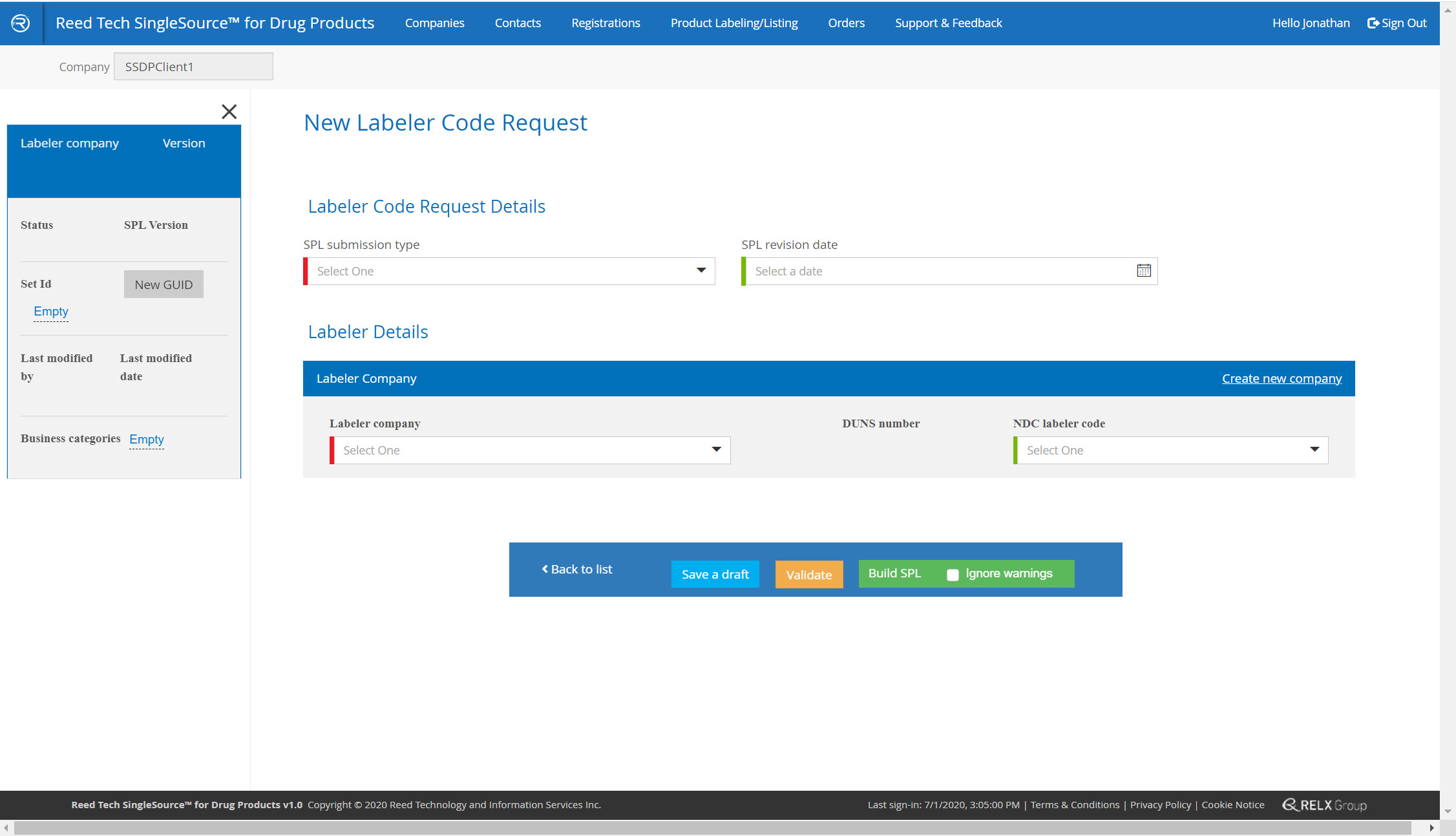
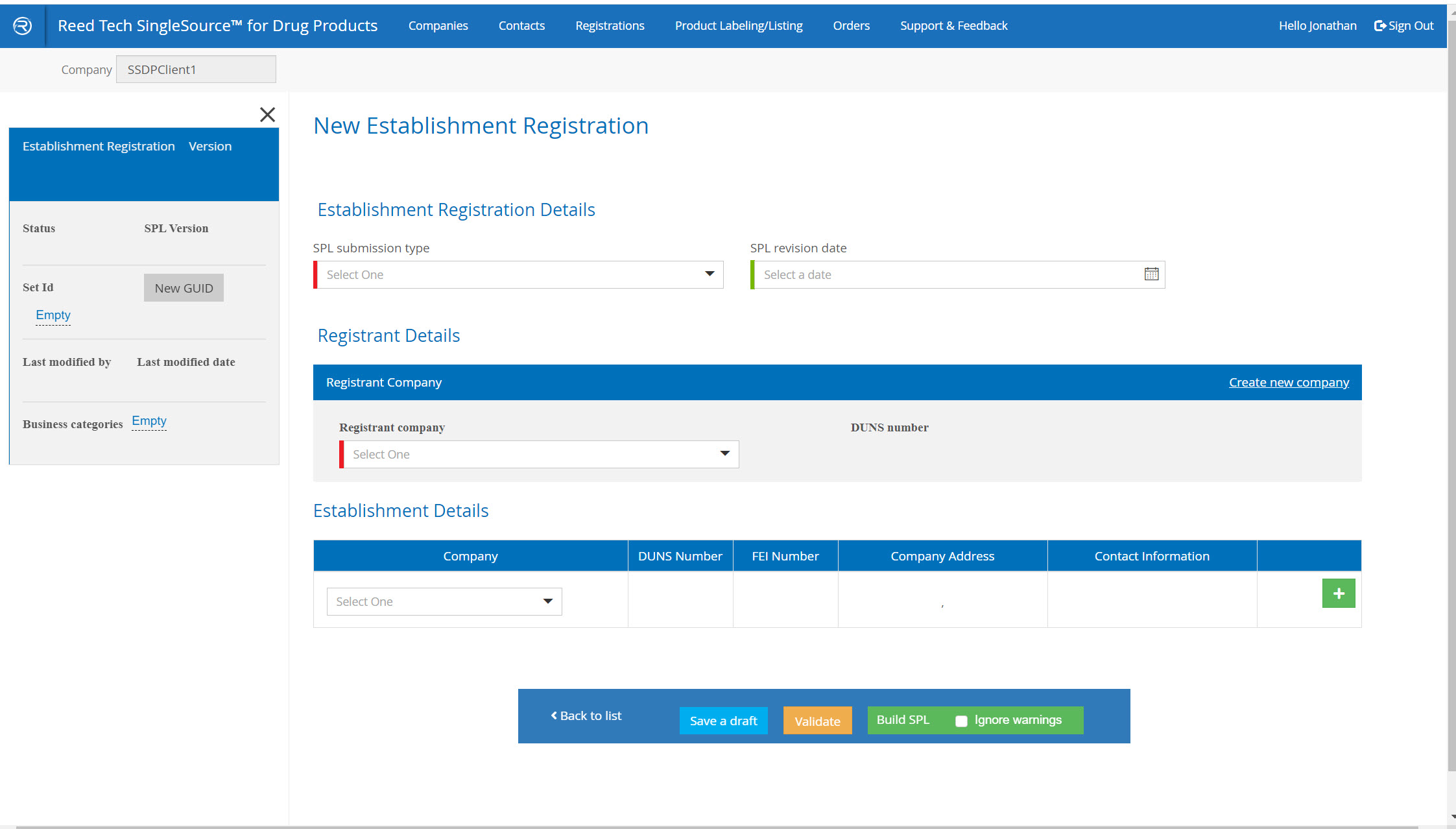
Labeler Code and Establishment Registration Enhancements
- Simple, guided system: you complete the form, validate it, and the system delivers the completed SPL in real time
- A complete database of your companies that are used in SPL listings
New labeler code form
New Establishment Registration Listings form
Better control and logs of your company data used in submissions
As you can see, these customer-driven enhancements have created an intuitive, user-friendly tool that should lessen the resources required to complete SPL submissions. We are excited to roll out our new features this fall and continue to add improvements based on additional customer feedback.
If you have any questions or to learn more, please reach out to our Pharma team at 215.557.3010 / [email protected] or request a demo here. Current customers can also request a link to recorded training sessions by contacting their Account Manager.