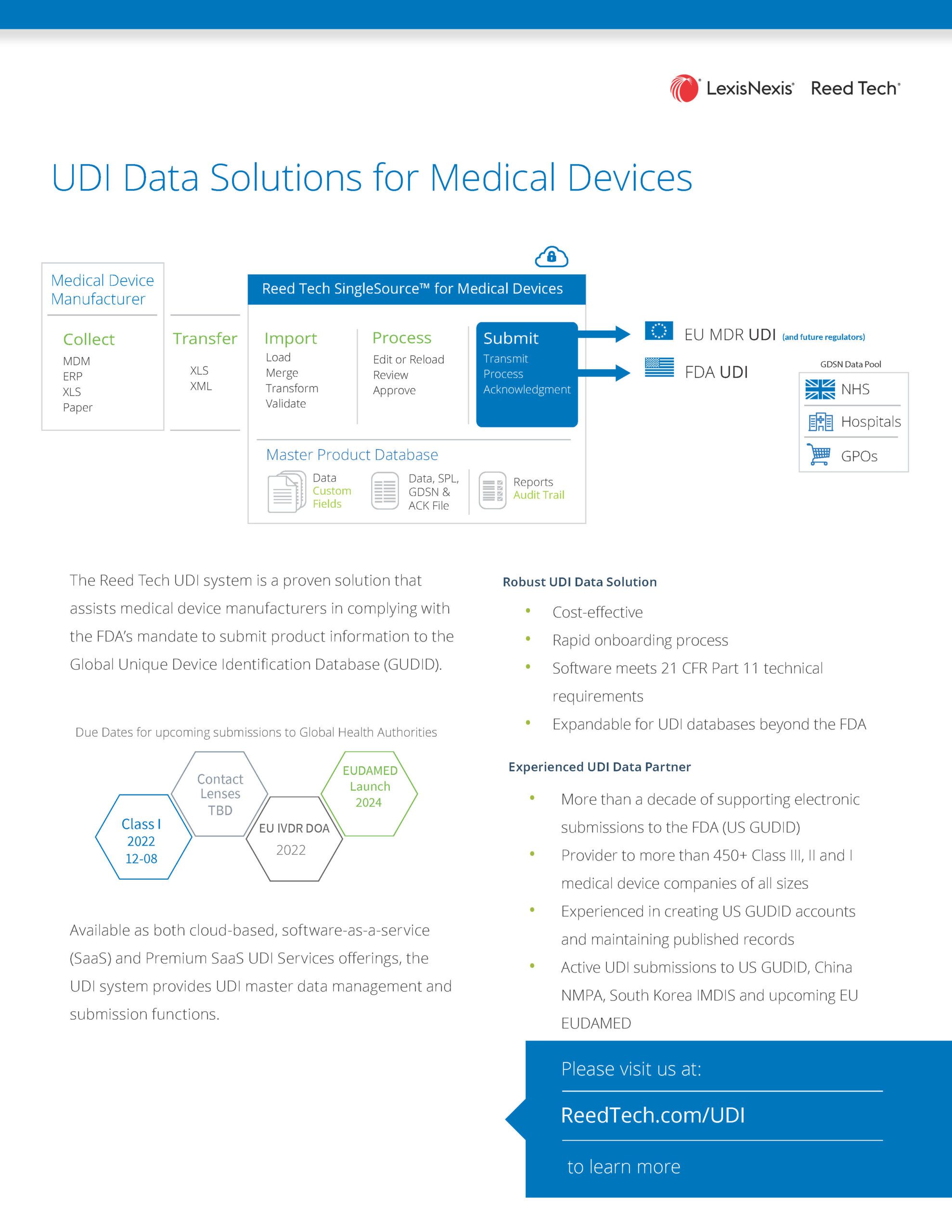
UDI Data Solutions for Medical Devices
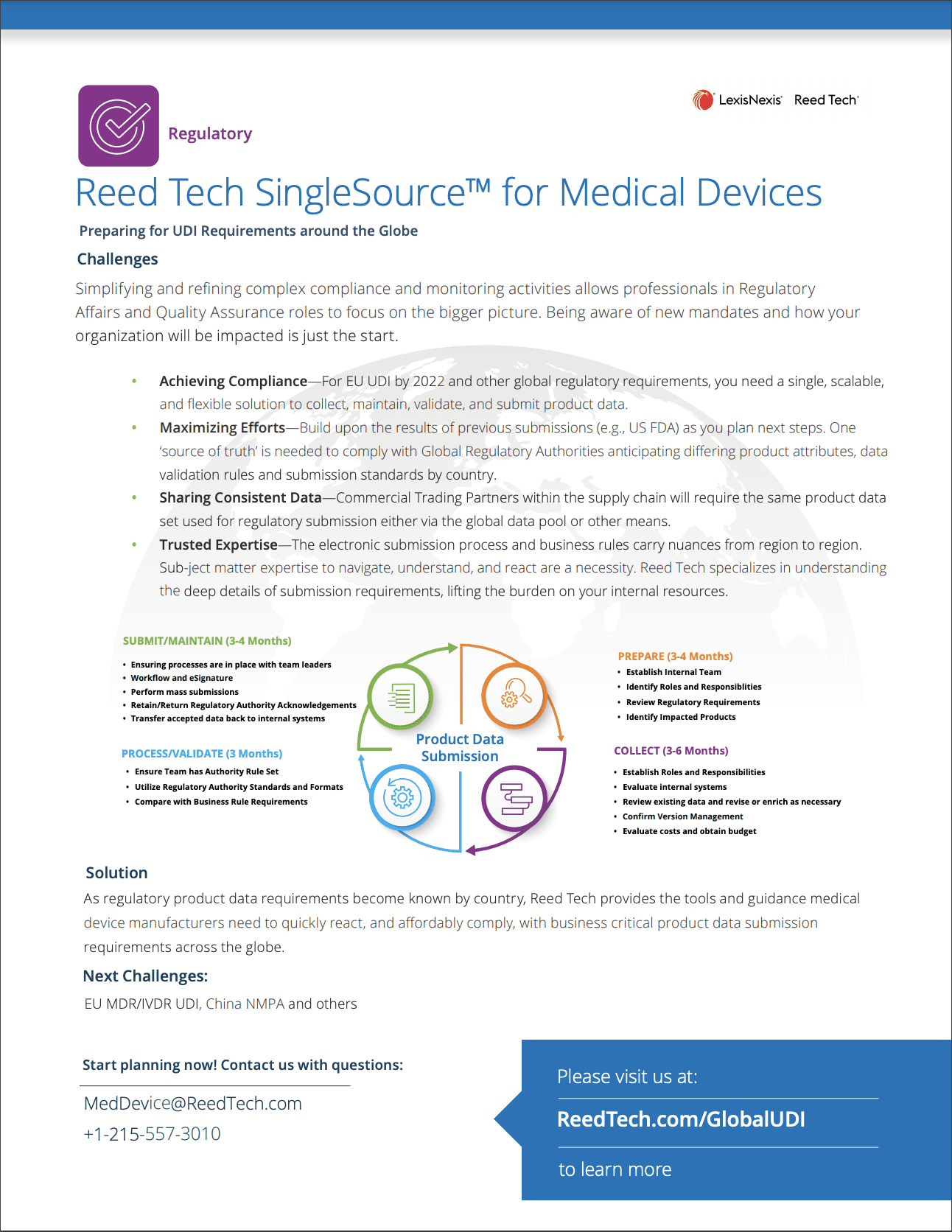
Reed Tech SingleSource™ for Medical Devices
UDI Data Solutions for Medical Devices
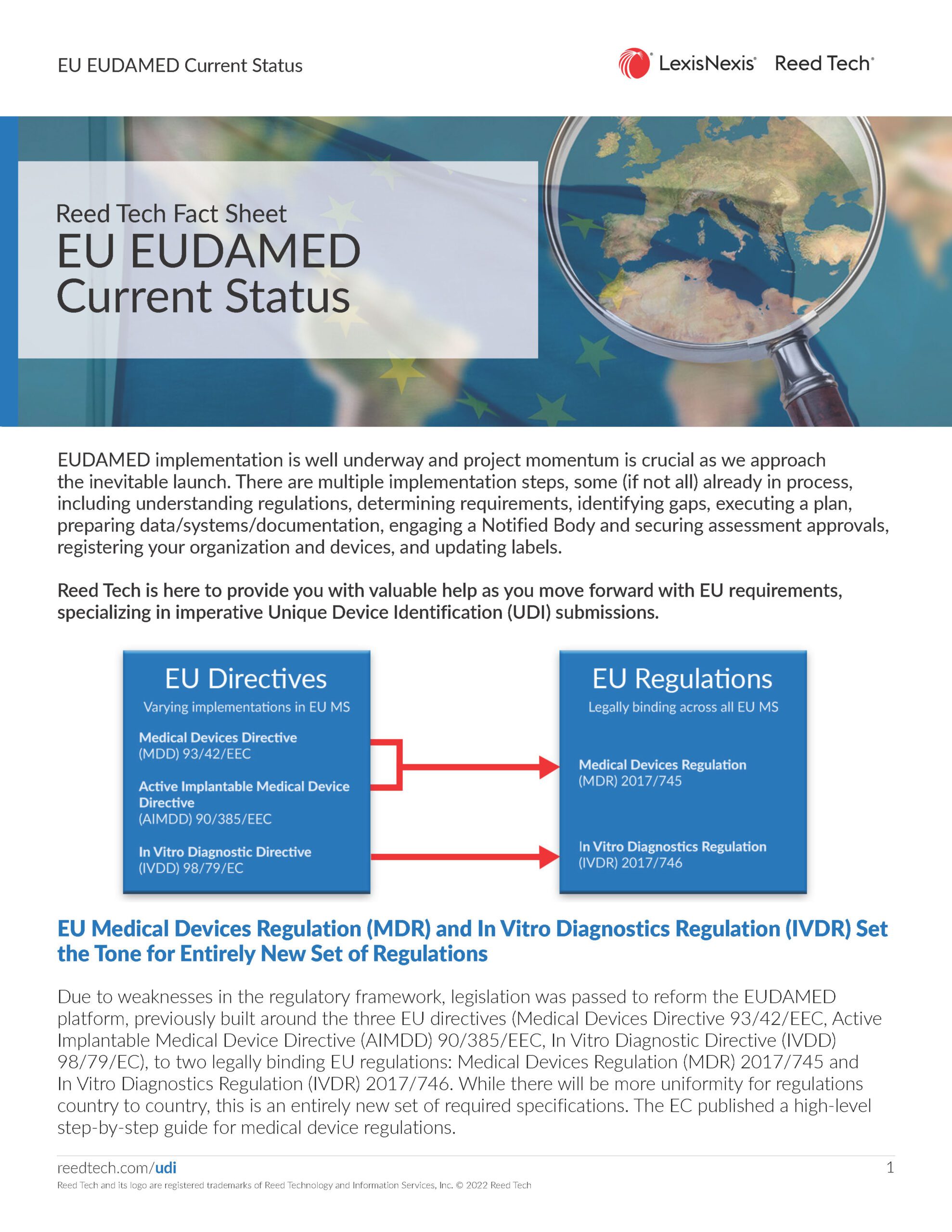
EU EUDAMED Current Status
Medical Device Terminology
Global UDI Summary of Health Authorities
South Korea – Intro to UDI
China NMPA – Intro to UDI
Electronic Common Technical Document (eCTD) Services
Modernization of Cosmetics Regulation Act (MoCRA)
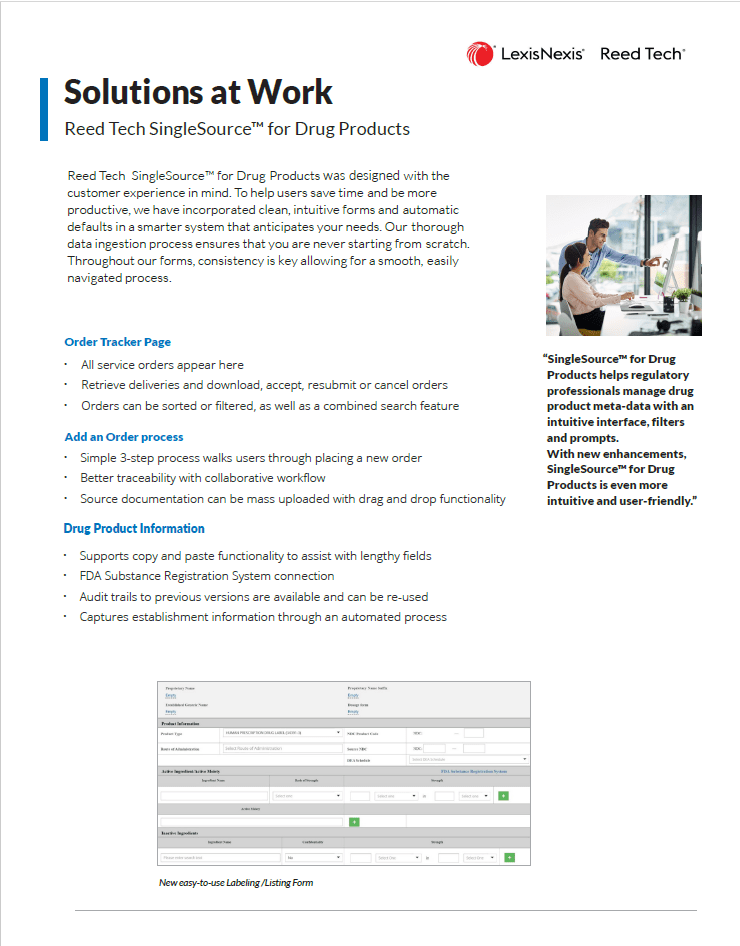
Reed Tech SingleSource™ for Drug Products
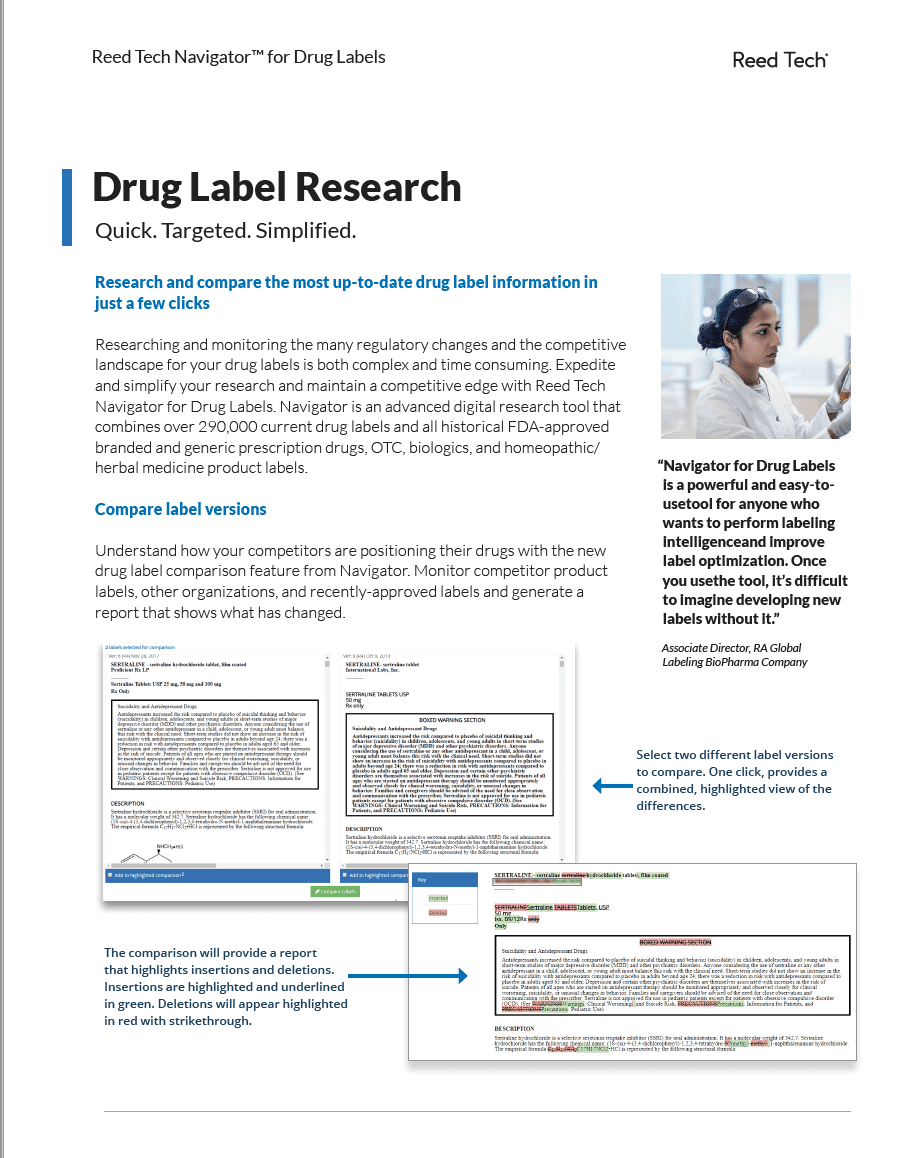
Reed Tech Navigator™ for Drug Labels
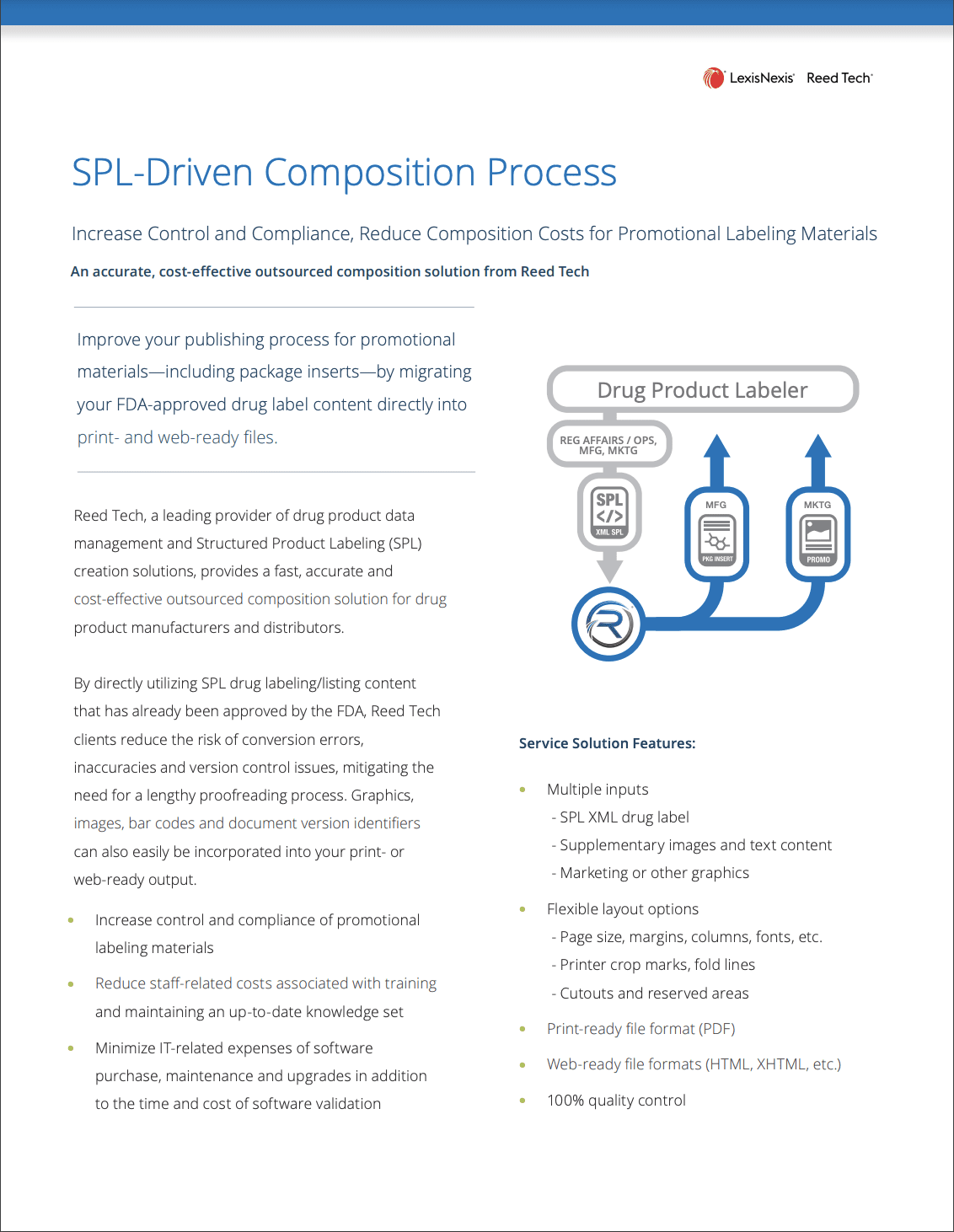
SPL-Driven Composition Process
SPL and eDRL Solutions and Services