Drug Label Research & Analytics
Reed Tech Navigator™ for Drug Labels
Preview Navigator™ for Drug Labels to learn how you can create accurate and up-to-date drug product content.
Navigator™ for Drug Labels combines US and centralised EU human drug label databases with advanced search, comparison, analysis and alerts tools
- Search nearly 500,000 drug labels
- Branded and generic prescription drugs
- Biologics and over-the-counter (OTC)
- Homeopathic and herbal products
- Set up alerts for updates in your Personal Library
Quickly and easily access the entire sets of FDA-approved prescription, over-the-counter (OTC) and homeopathic drug products and EU centralised prescription drug products
Navigator™ for Drug Labels helps pharmaceutical regulatory researchers ensure accurate and up-to-date drug label content.
Search the database using a single word or leverage up to seven categories and 90+ parameters to build a multi-dimensional search. Conduct side-by-side label comparisons of up to five labels to analyze similar products or previous label versions and discover acceptable usage changes. Monitor competitor products and recently-approved labels with LabelTrack, delivering personalized email alerts.
- Review up to 5 labels at a time
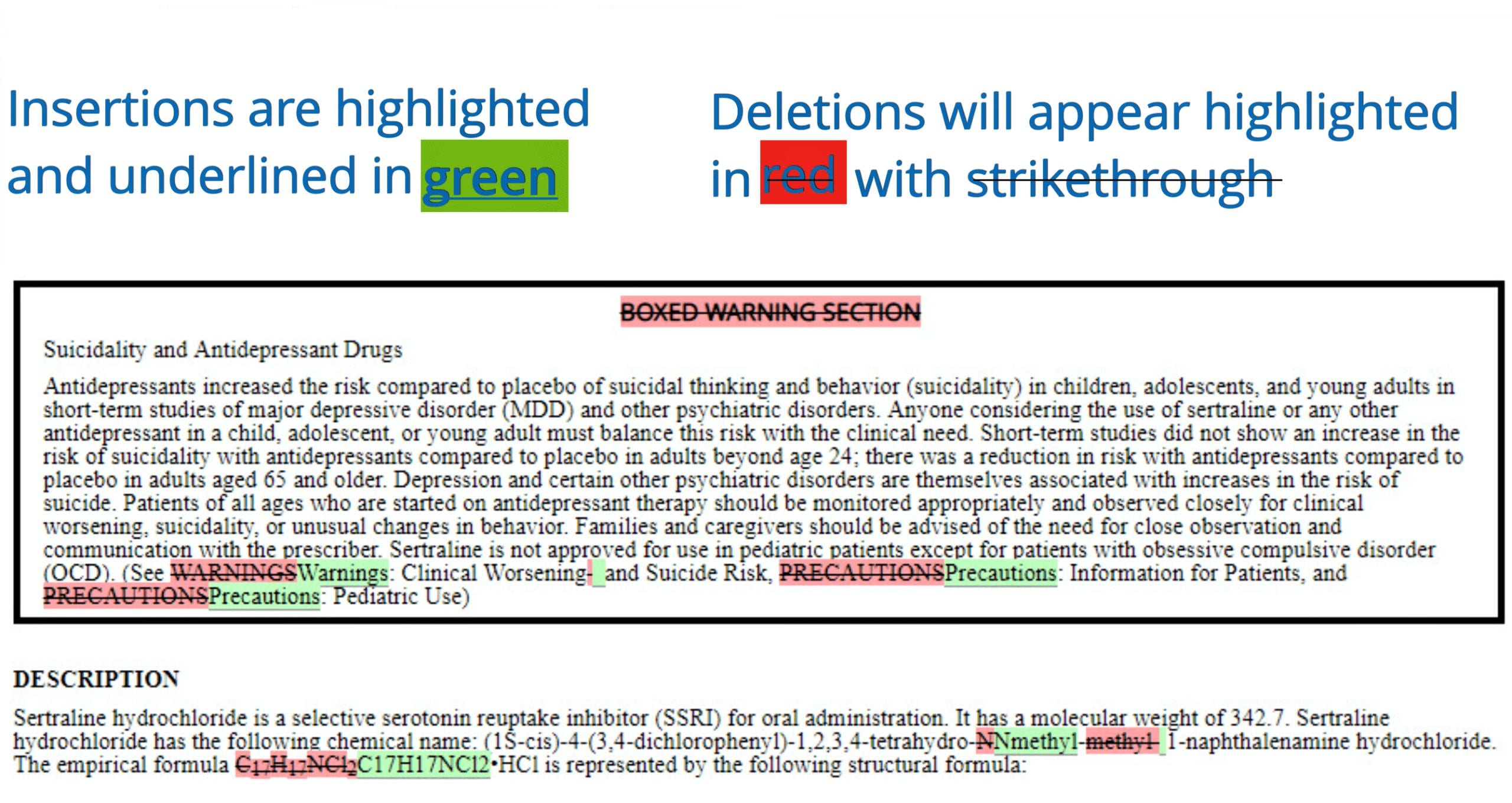
- Select 2 labels to compare and with 1 click see the highlighted view of differences
Navigator™ for Drug Labels Features
Regulatory Intelligence
Research and produce labels in line with corporate and industry standards.
- Conduct advanced searches of comprehensive US (Rx, OT, and homeopathic) and EU-centralised (Rx) human drug label databases
- Access nearly 500,000 historical and current drug labels, compare them side-by-side, and monitor them for changes
- Be confident in the most current, comprehensive information available with daily database updates
Streamlined Label Creation
Research and produce labels in line with corporate and industry standards
- Ensure global locations produce labels in line with corporate standards and industry standards
- Compare up to five labels at a time by section as well previous versions of a drug label
- Track label updates to ensure consistent data and formatting across multiple suppliers
Market Research
Bolster your market research efforts with insights into competitor products and reference drugs.
- Perform due diligence for acquisitions or new product development
- Gain actionable insights into competitors’ products
- Establish a competitive advantage
Case Development and Litigation
Research and procure documentation to support case development and litigation
- Support case development and due diligence
- Perform language analysis to determine acceptable communications
- Research language changes by label version
