FDA Drug Product Data Management
Reed Tech SingleSource™ for Drug Products
Centrally manage FDA human prescription, over-the-counter and animal drug product submissions data in Structured Product Labeling (SPL) format
SingleSource™ for Drug Products is a web-based application developed to 21 CFR Part 11 compliant standards that allows drug product manufacturers and distributors to collect and store their global product listing, establishment facility and labeler company data in an easy to use master submission data library.
How can Reed Tech SingleSource™ for Drug Products help?
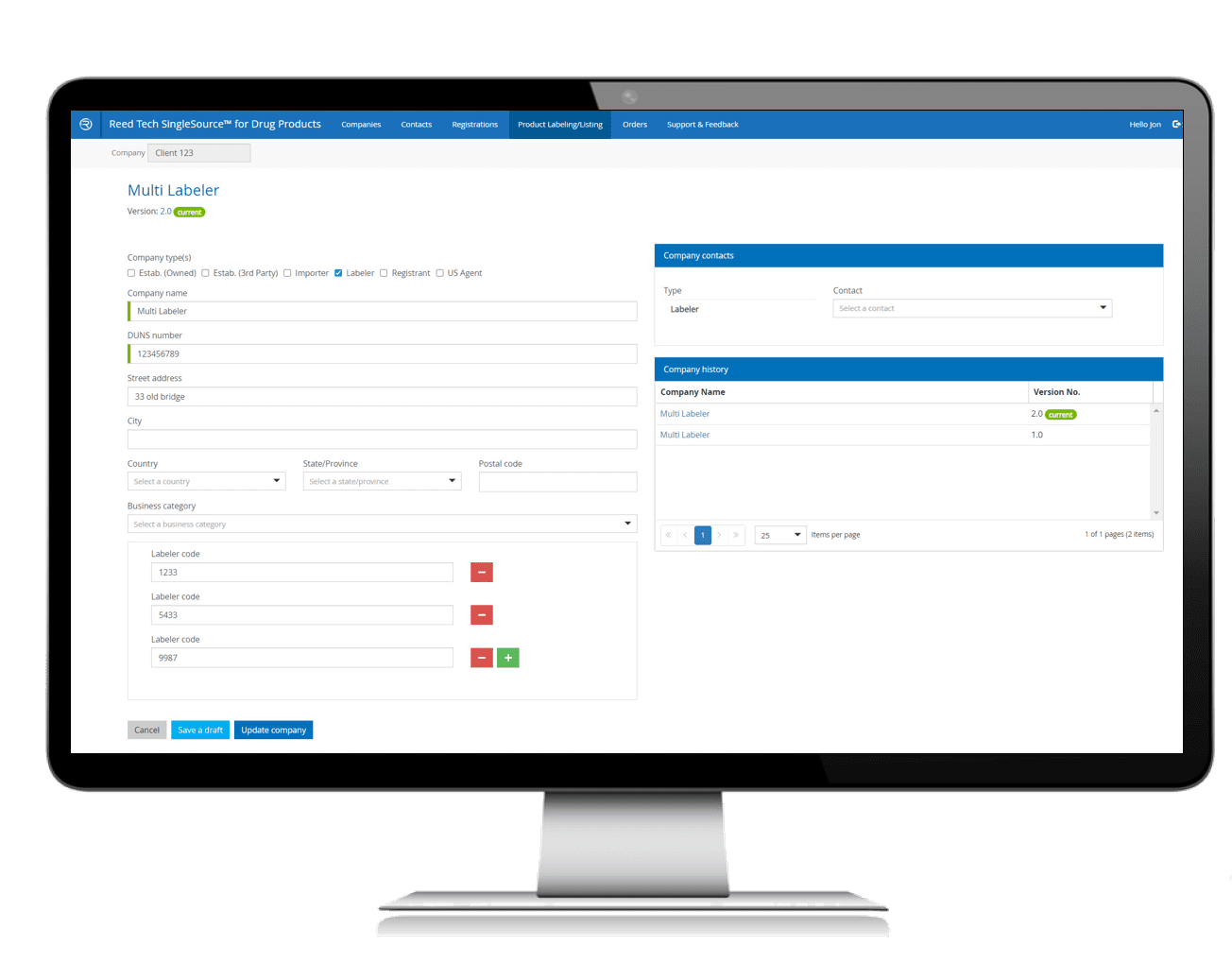
- Multi-Function Solution: SPL preparation and management helps meet strict FDA guidelines with data collection, capture and validation
- Leverage a single source of truth for product listing information, eliminating time searching databases that may span multiple departments or facilities
- Available 24/7 in a Software-as-a-Service model without the need to install or validate software helps lower IT cost and the need for third party support
- Data validation based on up-to-date FDA business rules and industry controlled vocabularies ensures data accuracy
- Standardized SPL conversion request template, allows for quicker order processing and placement into the Reed Tech production process
- Ensures consistent usage across all users in a simple, guided system
Enter data once, use many times
SingleSource™ for Drug Products provides on-demand HL7 Structured Product Labeling (SPL) format generation for all human prescription, over-the-counter, dietary/herbal medicines and animal drugs, allowing you to meet strict FDA deadlines without requiring third-party support or premium costs.
Easy-to-use web-based SPL forms include built-in FDA business rule verification for quick identification and resolution helping to remove ambiguity around the accuracy of your data. Once your data is imported or entered into the system, you can use it many times with peace of mind that your data is accurate and meets FDA requirements.
SingleSource™ for Drug Products Features
Secure Regulatory Data Management
- Store product data in a secure cloud repository
- Orders can be filtered, sorted or found in a combined search feature
- Retrieve deliveries and download, accept, resubmit or cancel orders
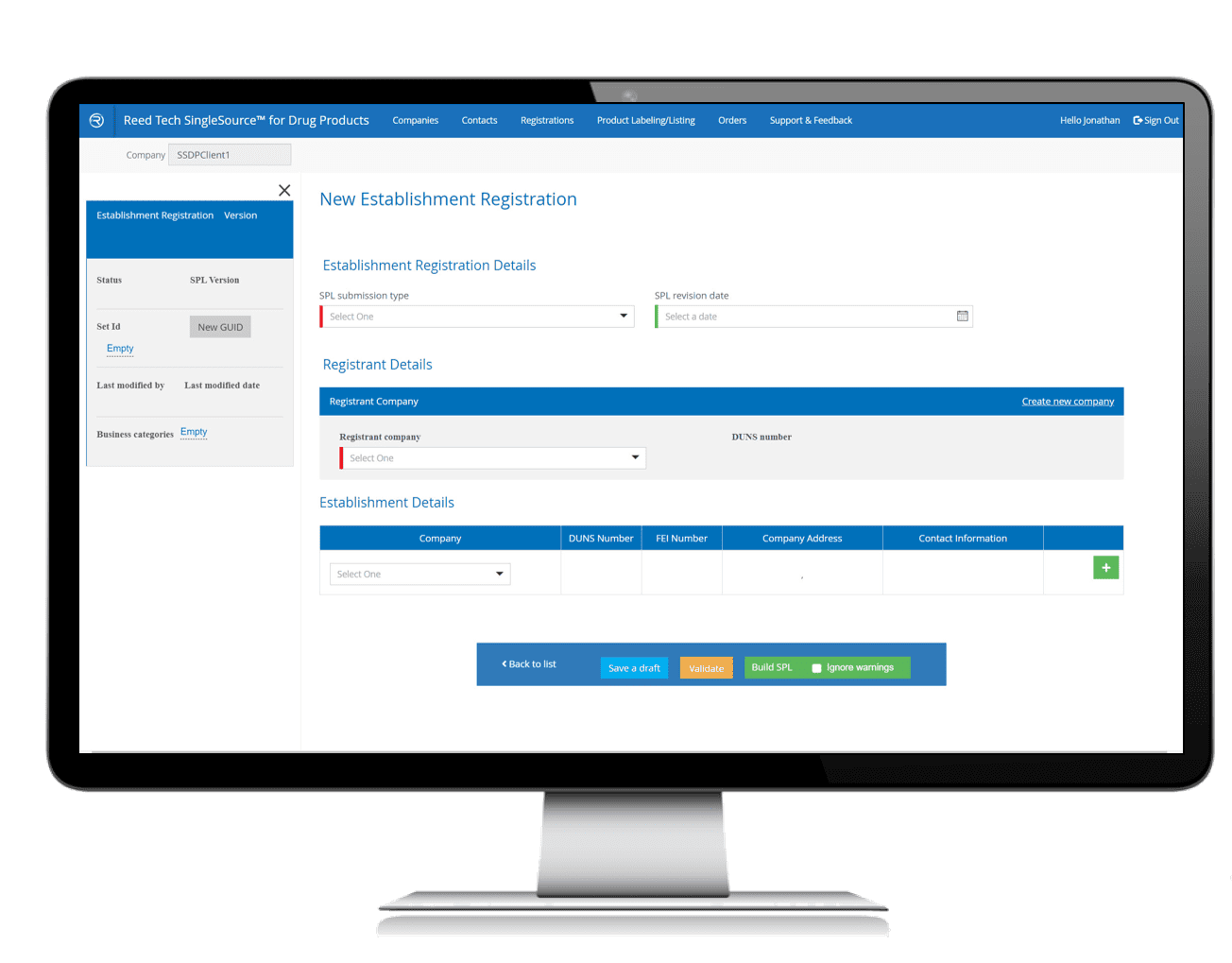
Streamlined SPL Conversion Process
- SPL creation, validation and management in one
- Reduces time to submission
- Improves processes for cross-functional teams
Master Data Management
- Data management for drug product, establishment facility and labeler company data
- On-demand SaaS SPL generation for SPL-ER, SPL-LCR and Product Listing Metadata updates
Transparent and Collaborative Workflow
- View into progress of work for all users
- Facilitates meeting internal and FDA deadlines
- Filters and prompts integrated for a seamless, guided process
Speak to a Subject-Matter Expert
SingleSource™ for Drug Products was built to meet the product data management needs of pharmaceutical companies. See what it can do for you.
Sign up for a demo today.

