LexisNexis Reed Tech
SPL & XML Services for Rx, OTC & Biologics
55k+ SPL electronic drug labeling submissions filed with the FDA
Simplify electronic drug & establishment listing compliance for drug and biologic products
LexisNexis Reed Tech serves more than 1,000 drug product manufacturers and distributors with solutions and services to manage product listing, establishment facility and labeler company data across multiple teams with a secure and validated cloud-based solution.
Electronic Drug Registration and Listing (eDRL)
Ensure regulatory compliance. Our experts can remove confusion around SPL and XML formatting. Meet FDA expectations and prevent technical issues or errors that extend your submission process. Manage product listing, establishment facility and labeler company data across multiple teams with a best-in-class solution.
Electronic Common Technical Document (eCTD)
Reed Tech now offering eCTD services to our Pharmaceutical customers.
The Electronic Common Technical Document, commonly referred to as eCTD, is a standard format for submitting regulatory applications, amendments, supplements and reports to Global Health Authorities. eCTD is structured into five modules of information and data relating to a medicinal product and allows for electronic submission from applicant to regulator using an XML backbone as a navigation file and for effective version control.
eCTD & Consulting Services
- eCTD strategy
- eCTD project management & compilation
- Experienced validation troubleshooting
- 100% validated submission transmission to FDA, EMA, CESP, MHRA etc.
PDF & MS Word Publishing
- ICH & regional regulatory authority standards & best practices
- MS word formatting & PDF publishing
- Clinical Study Reports
FDA Conversion and Submission Services

- Drug labeling and listing for Rx, Veterinary, OTC, Homeopathic and Biologic Products
- Annual Blanket-No Change Certification
- NDC/NHRIC Labeler Code Request/Registration Establishment Registration and annual maintenance
- GDUFA Self-Identification
- ACA 6004 Drug Sample Distribution Reports
- Lot Distribution Reporting (LDR)
- Electronic Submissions Gateway (ESG)
- Templates for media output for downstream use of SPL-XML for product use documentation, patient or marketing materials
Health Canada XML Product Monograph
Health Canada will start to accept drug monographs in XML format on a “by request” basis April 1 – July 31, 2020, with voluntary launch to begin by Spring 2021.
Reed Tech knows the Canadian system first hand. For the past two years, we have been working in partnership with two large international drug product manufacturers, in pilot activities with Health Canada, to help establish and test its new XML PM program.
We have provided XML Structured Product Labeling (SPL) services for US FDA drug labeling to 1,000+ pharmaceutical companies since 2005. We can support your conversions of product monographs to the XML PM format as required by Health Canada.

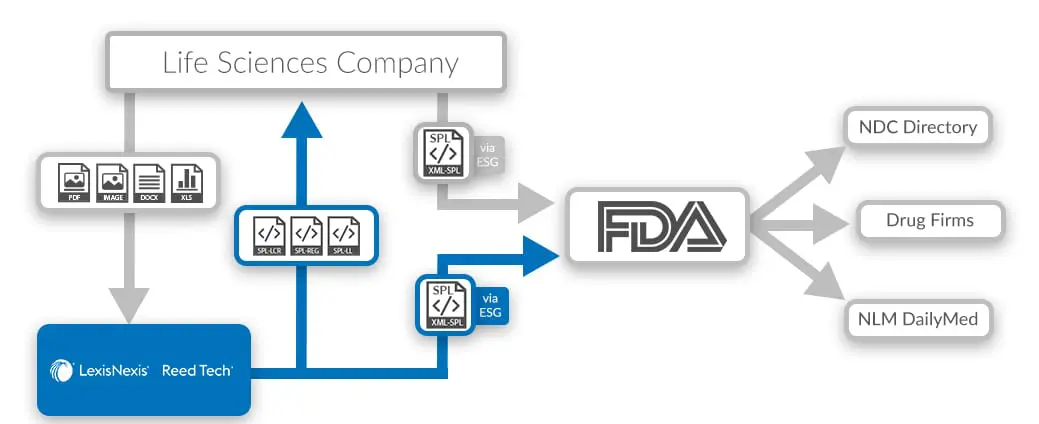
FDA SPL Composition Process
Gain increased control and create print and web-ready files directly from SPL. With labeling composed in XML, Reed Tech offers a process to use the Content of Labeling portion of the FDA-accepted SPL Drug Listing file as the source for:
- Manufacturing Package Insert (PI)
- Marketing media-for print or advertising use
- Med Guide
- Patient Package Insert (PPI)
- Product Web Pages
What is SPL?

Structured Product Labeling (SPL) is a Health Level Seven (HL7) International standard for regulatory guidance documents as a method for communicating product and facility information. Accepted by the Food and Drug Administration, SPL enhances the cohesiveness and honesty of product information because it requires reliable structure and standardized terminology. SPL documents consist of not only the content of labeling (text, figures, and tables) but also information about the product (drug listing data elements) that is readable by machines.
The SPL specification was created to assure that there was a consistent way to develop labeling content. By using an established method for product labeling, enhancements and improvements can be attained throughout every step of the process, from the creation to the distribution of labeling content by industry and health authorities. The labeling of products is a crucial part of product life cycle management.

The procedure of product labeling is detailed, complicated, and closely monitored. The content that the product label contains is very precise, especially regarding its safety data and adverse effects. With so many parties within the healthcare system relying on accurate information, it is problematic for one party to misinterpret another’s message. SPL solves for clear communication with regulated classifications. Many FDA divisions have required SPL since June 2009, including Biologics (CBER), Veterinary (CVM), Office of Nonprescription Products (ONP), and Medical Devices (CDHR). Other divisions, like the Center for Drug Evaluation and Research (CDER), have followed the requirement since October 2005.
