US FDA Class I UDI Submission Deadline
UDI Product Data is now due for specific scenarios (Enforced as of December 8, 2022)
Class I and Unclassified medical devices that are required to be labeled with a UDI code, must submit product data to the FDA GUDID now as the enforcement date began December 8, 2022.
Note that the FDA has provided a Final Guidance outlining exceptions for “Consumer Health Products,” i.e., certain Class I devices that are 510(k) Exempt and only sold OTC directly to consumers. (Guidance finalized on July 22, 2022, previous enforcement date for UDI submissions was September 24, 2022.)
Number UDI records Reed Tech has submitted to GUDID
If you market your Class I device according to one or more of the following conditions, the Class I GUDID Submission Exception does NOT apply and you must report product data to the FDA GUDID.
- You distribute your Class I device to professional healthcare facilities (hospitals, clinics, physician offices, etc.)
- Your Class I device is intended only for use by healthcare professionals
- Your Class I device is reused on different patients and reprocessed using high-level disinfection and/or sterilization
- Your Class I device requires 510(k)
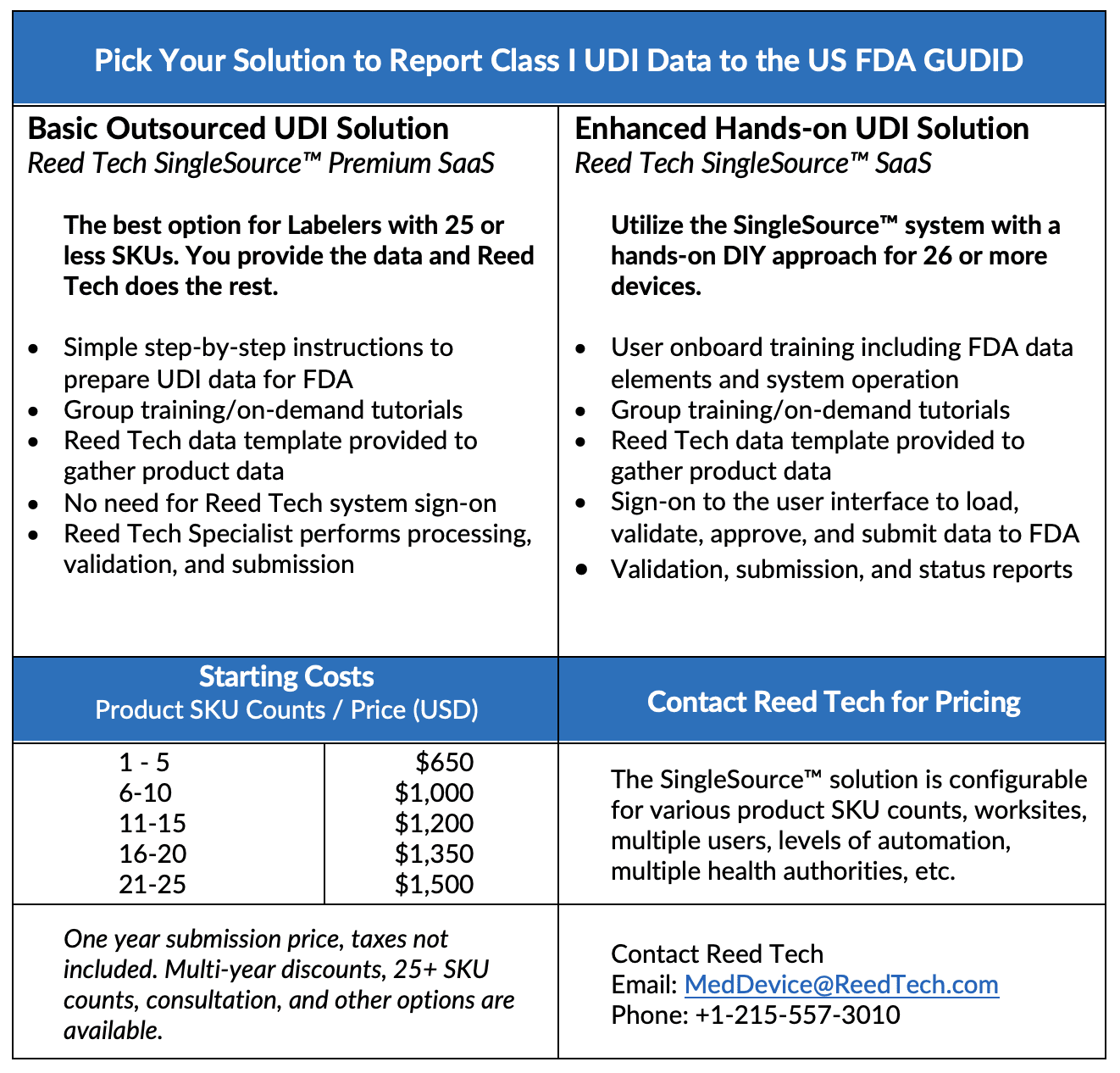
Submitting UDI product data to a Health Authority can quickly become overwhelming. We can help.
Reed Tech can efficiently help you achieve compliance and relieve your team of this regulatory burden of reporting your Class I devices, Unclassified devices, and any outstanding Class III or II devices to the FDA GUDID. The Reed Tech SingleSource™ solution enables you to quickly and affordably comply with regulatory product data submission requirements. It provides a single, scalable, and flexible data management platform that allows you to collect, maintain, validate, and submit product UDI data to the FDA and other global health authorities as necessary.
Reed Tech can help you with a fast start up and dedicated support to gain peace of mind:
- Simple step-by-step instructions to prepare UDI data for FDA
- A full team of highly specialized experts efficiently processing, validating and submitting your data to the FDA with FDA acknowledgement notice
- US CFR Part 11 compliant
- Free up time from resources to work on other time-sensitive activities
