Analyze Medical Device Safety, Quality and Regulatory Data
Reed Tech Navigator™ for Medical DevicesNavigator™ gives you the power to analyze regulatory data
-
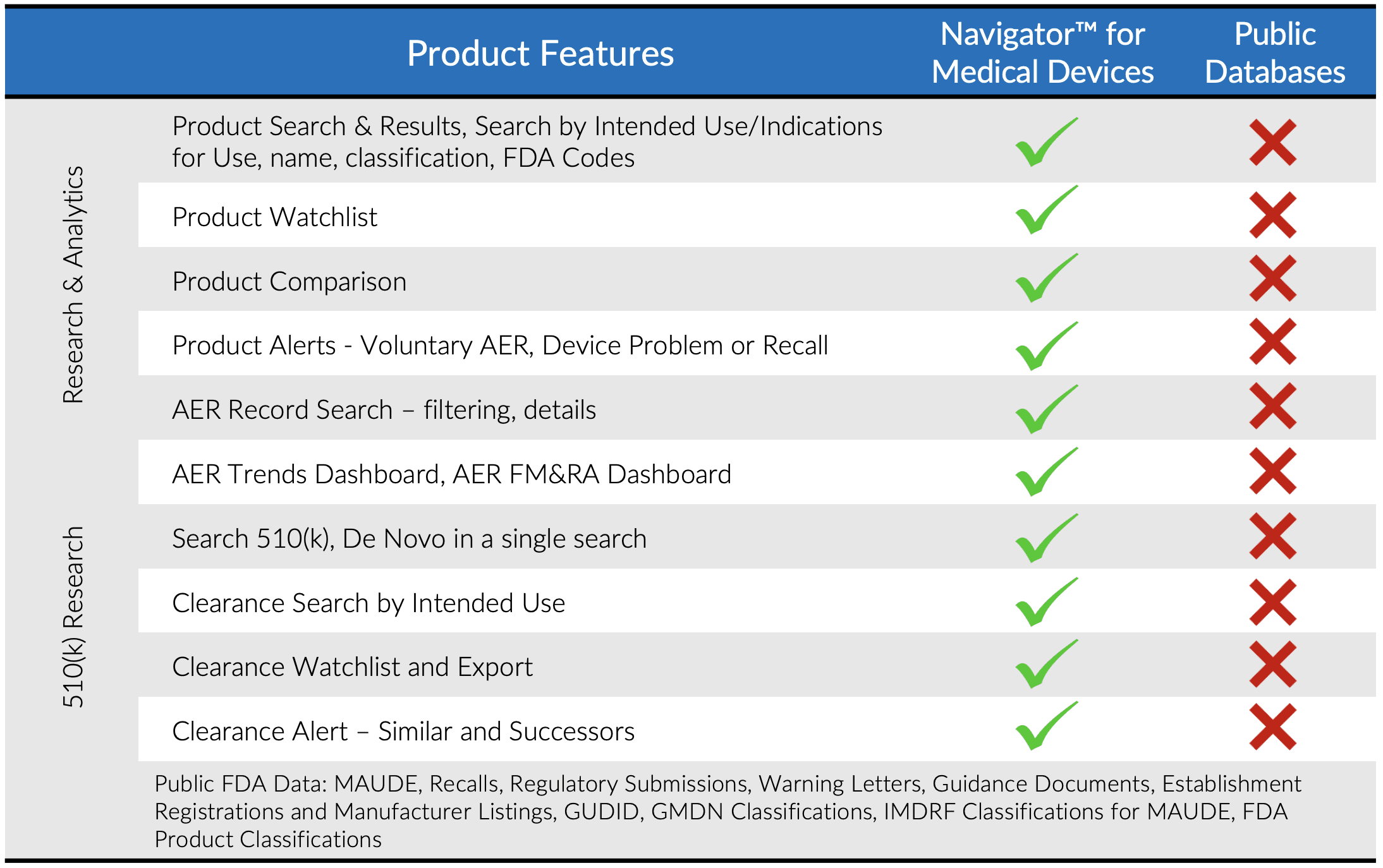
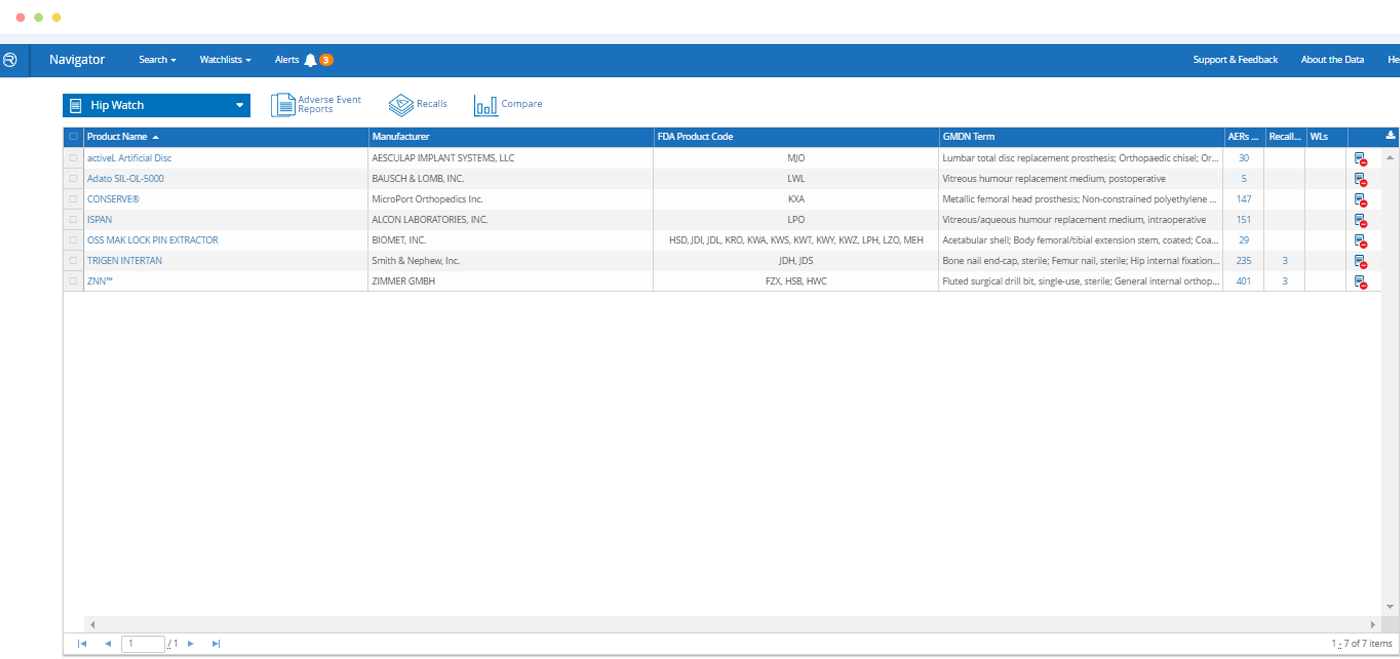
- Search a comprehensive database of companies, products and safety events from FDA and other publicly available sources
- Database is cleansed and normalized to link companies, products and events
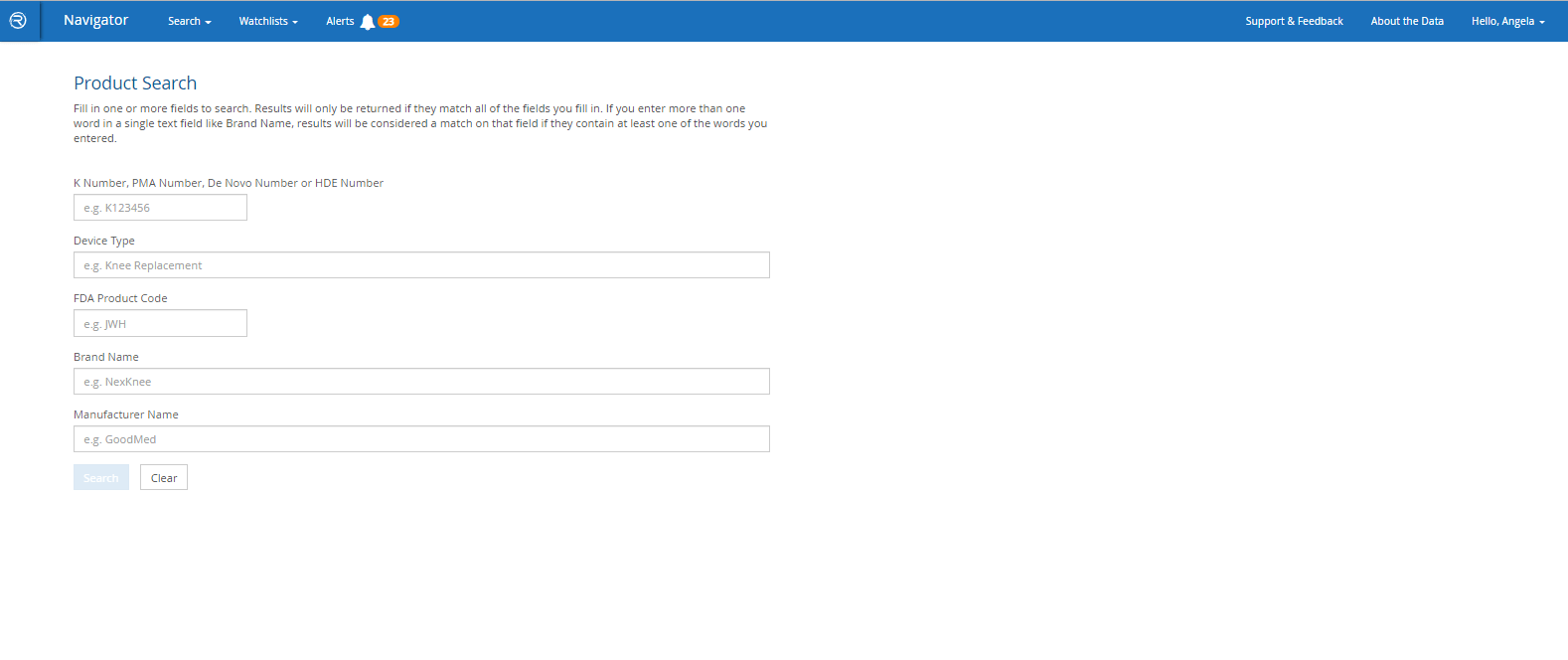
- Search by FDA product codes, brand and manufacturer names, intended use / indications for use, key word and other criteria
- 27,000+ Manufacturers and establishments
- 129,000+ Class II and III medical device products
- 173,000+ FDA Clearances and Approvals
- 24,000+ Recalls
- 9,500,000+ Adverse Event Reports
- Updated with new data monthly
*Search by intended use/indications for use is a feature only found in Navigator.
How does Navigator™ make regulatory research more efficient?
Multiple filtering and criteria options make search and retrieval in Navigator superior to the limitations of most publicly available databases.
Would you like to learn more about competitors or industry standards when it comes to adverse event reports or recalls for a specific medical device or category of devices? Do you need to find an appropriate 510(k) for a new product application? Is due diligence required to research trends for a specific legal case? *Do you need to pin-point specific products based on their intended use or indications for use?
Set Watchlists and alerts and find the answers in Navigator.
Get started with Navigator™
Single User 510(k) Research
fromSingle User Research + Analytics
fromResearch + Analytics + 510(k)
most popular choiceMonthly pricing based on one-year subscription. Individual customization and minimum three-month subscription length available. Taxes not included.
Unified Product View
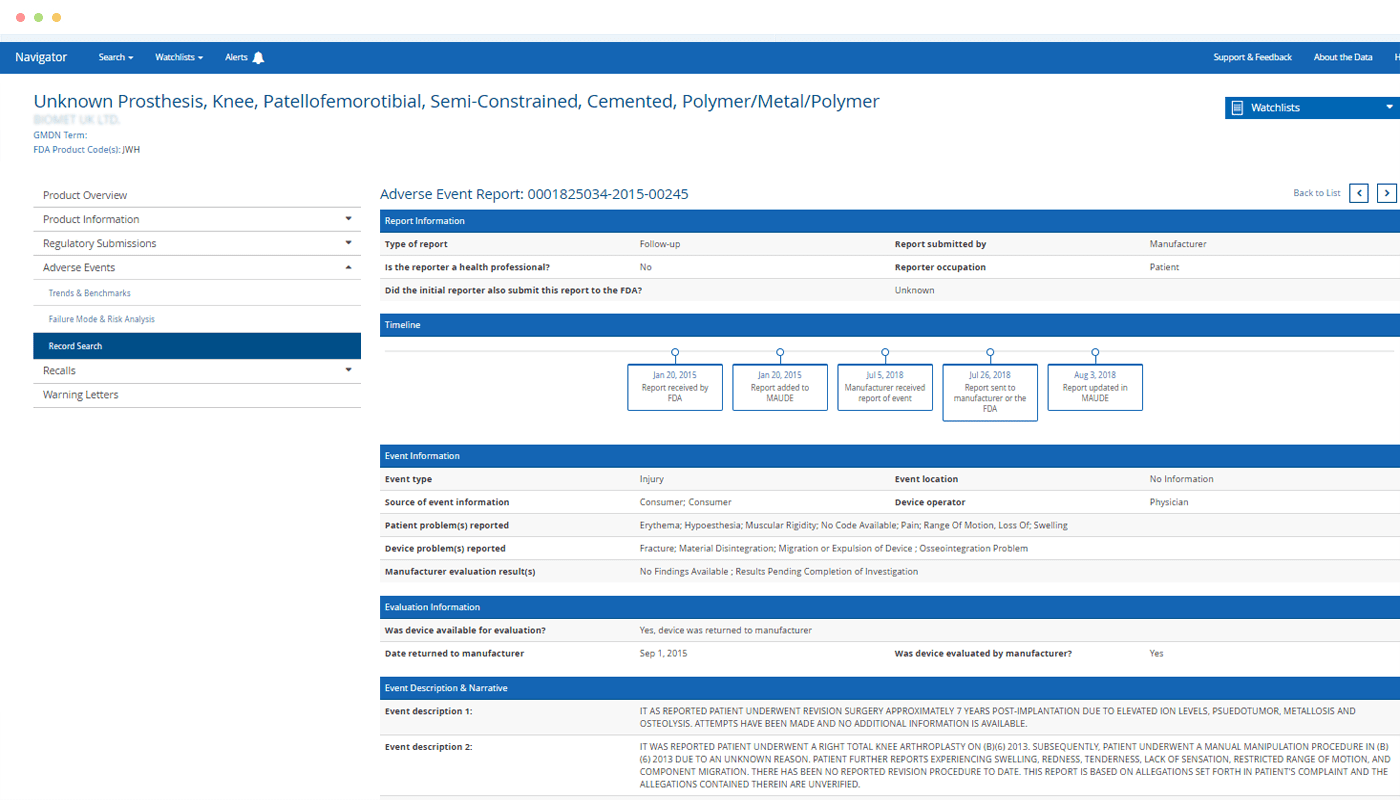
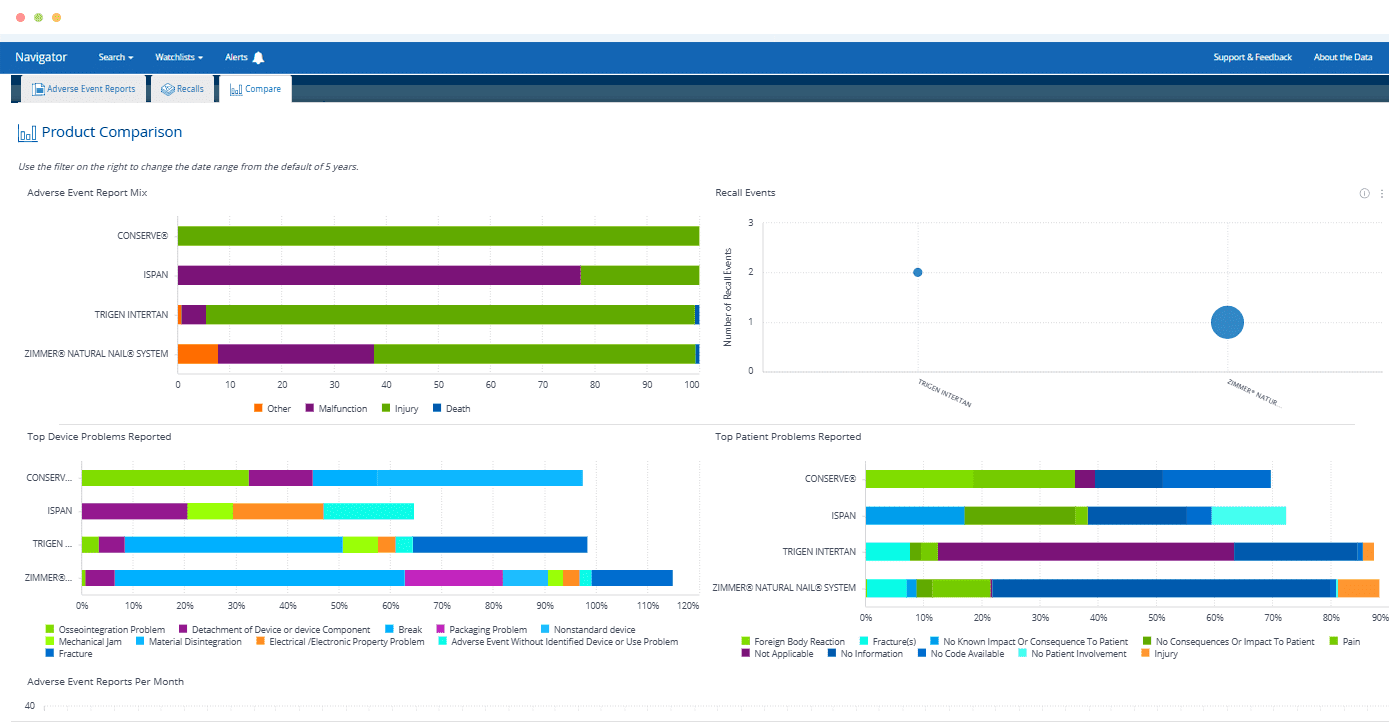
Eliminate frustration and save hours compared to searching for risks related to a particular product across the FDA’s disparate databases.
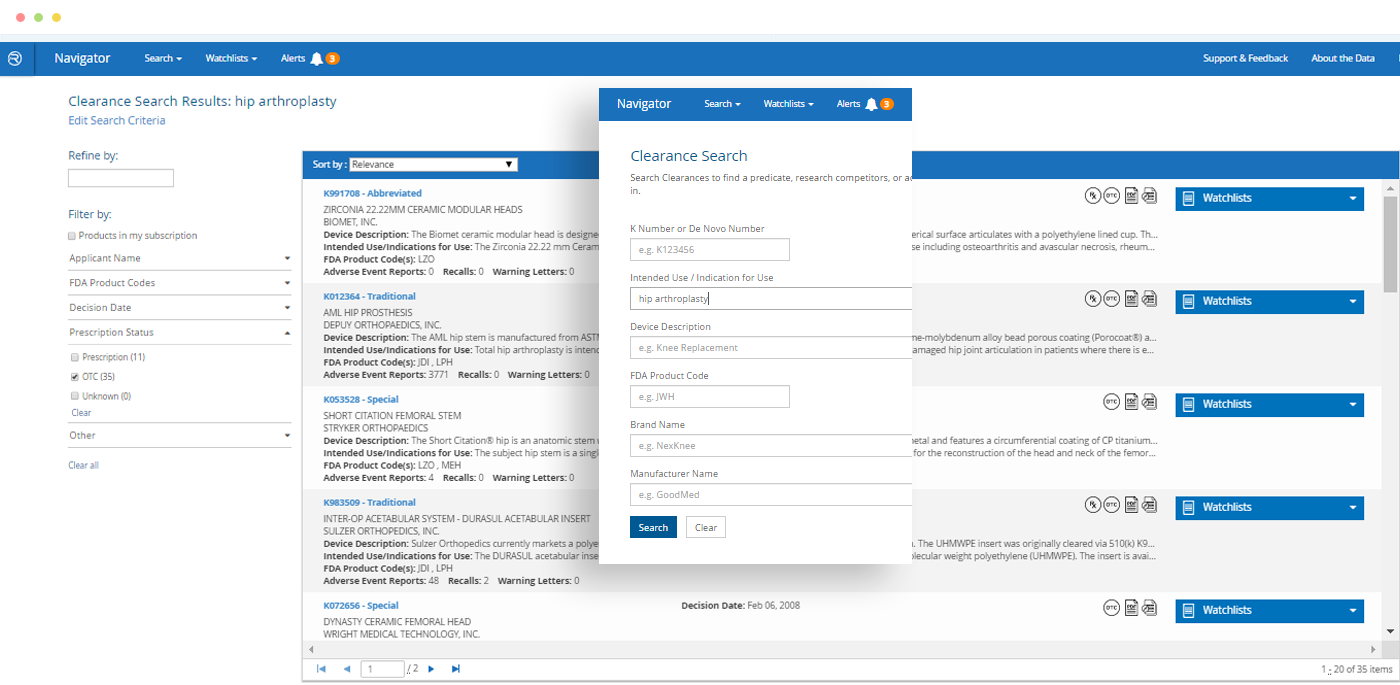
Smart Search
Quickly find a specific product – or find all products of a particular type – and see at-a-glance which has the most adverse event reports, recalls or warning letters.
Watchlists – Products and/or FDA Clearances
In just a few clicks, build a list of products you want to monitor on an ongoing basis for new information.
Predicting Medical Device Safety Recalls Using Publicly Available Data
Navigator™ for Medical Devices is a unique, best-in-class solution designed to help:
Law Firms
Find the best predicates to expedite your 510(k) applications and discover and monitor safety events.
Consultants
Easily identify predicate devices to bring a new device to market through the FDA’s 510(k) application process.
Investors
Find the best predicates for your client’s 510(k) applications and discover and monitor safety events.
Manufacturers
Find predicate and successor devices and simplify your post-market surveillance efforts.
Hospitals
Monitor your inventory for new safety events and compare quality of devices before purchasing.
Insurers
Quickly identify product liability using data analytics to compare safety profiles of like devices.
“The more I search and filter in Navigator, the more impressed I am with the depth of insight it provides. There’s nothing else like it for trending Adverse Event Reports and quickly seeing an individual product as compared to the industry in the criteria of your choosing. The utilization of all the filtering possibilities, including the use of the FDA product codes and GMDN coding enables on-point reporting you can’t get anywhere else.”
– Mark Wasmuth, CEO, GMDN Agency
Knowledge Center

Need a more customized package?
Contact us for a subscription built for you!