Medical Device Resource Center for Reed Tech Life Sciences
INTERNAL USE ONLYINTERNAL USE ONLY
This internal resource center is a hidden page for Life Sciences Sales to reference to further educate customers about Reed Tech expertise, UDI implementation, UDI data solutions for medical devices, global health authorities and more.
How to Use This Page
Please note, this webpage is hidden and is for internal purposes only. To provide customers with these resources, only copy and paste these links into emails or other correspondence.
Do not send this webpage to external users.
Helpful Links
About Reed Tech
Reed Tech Medical Device Solutions
- Medical Devices Brochure
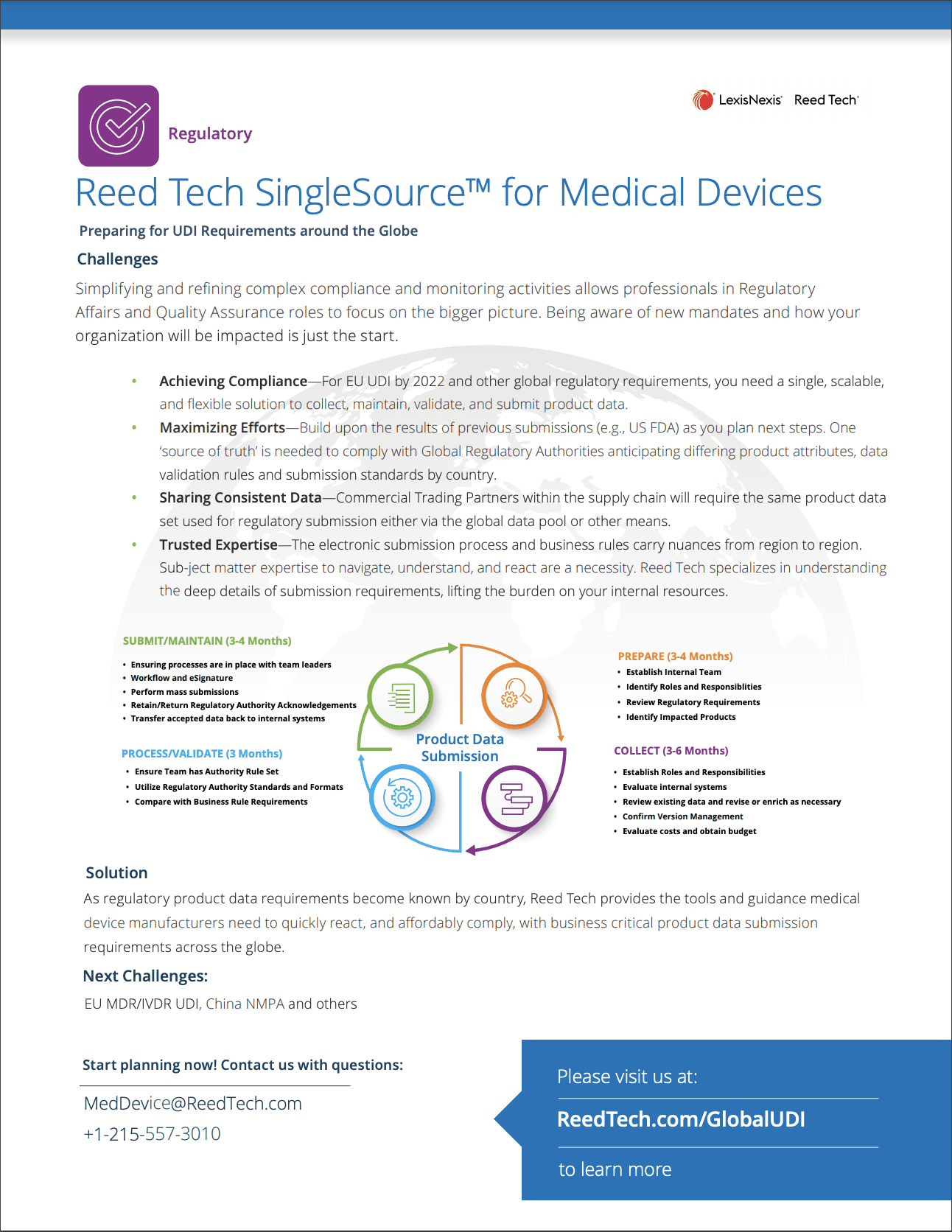
- Reed Tech SingleSource™ for Medical Devices
- Reed Tech SingleSource™ Focused Expertise
Unique Device Identification (UDI)
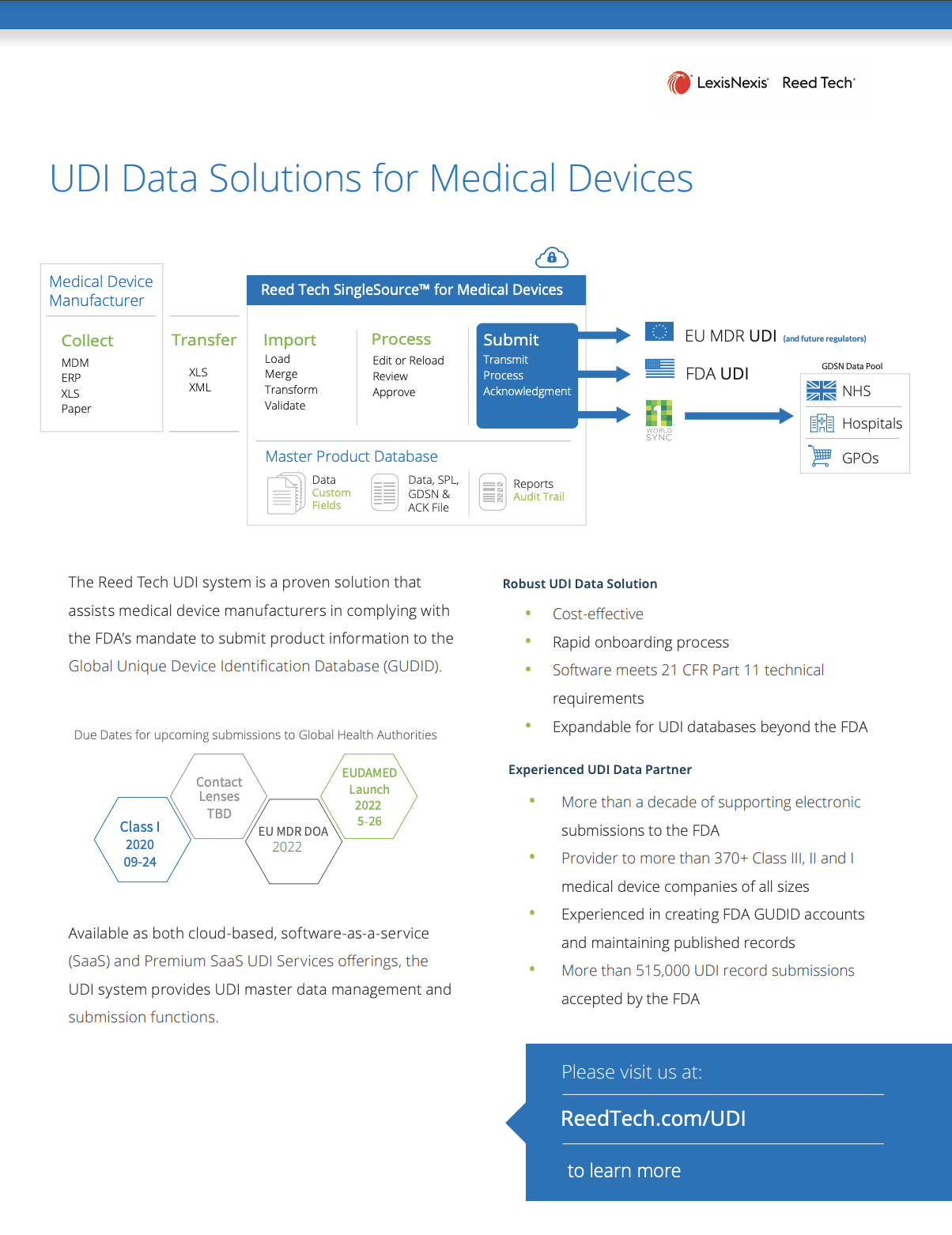
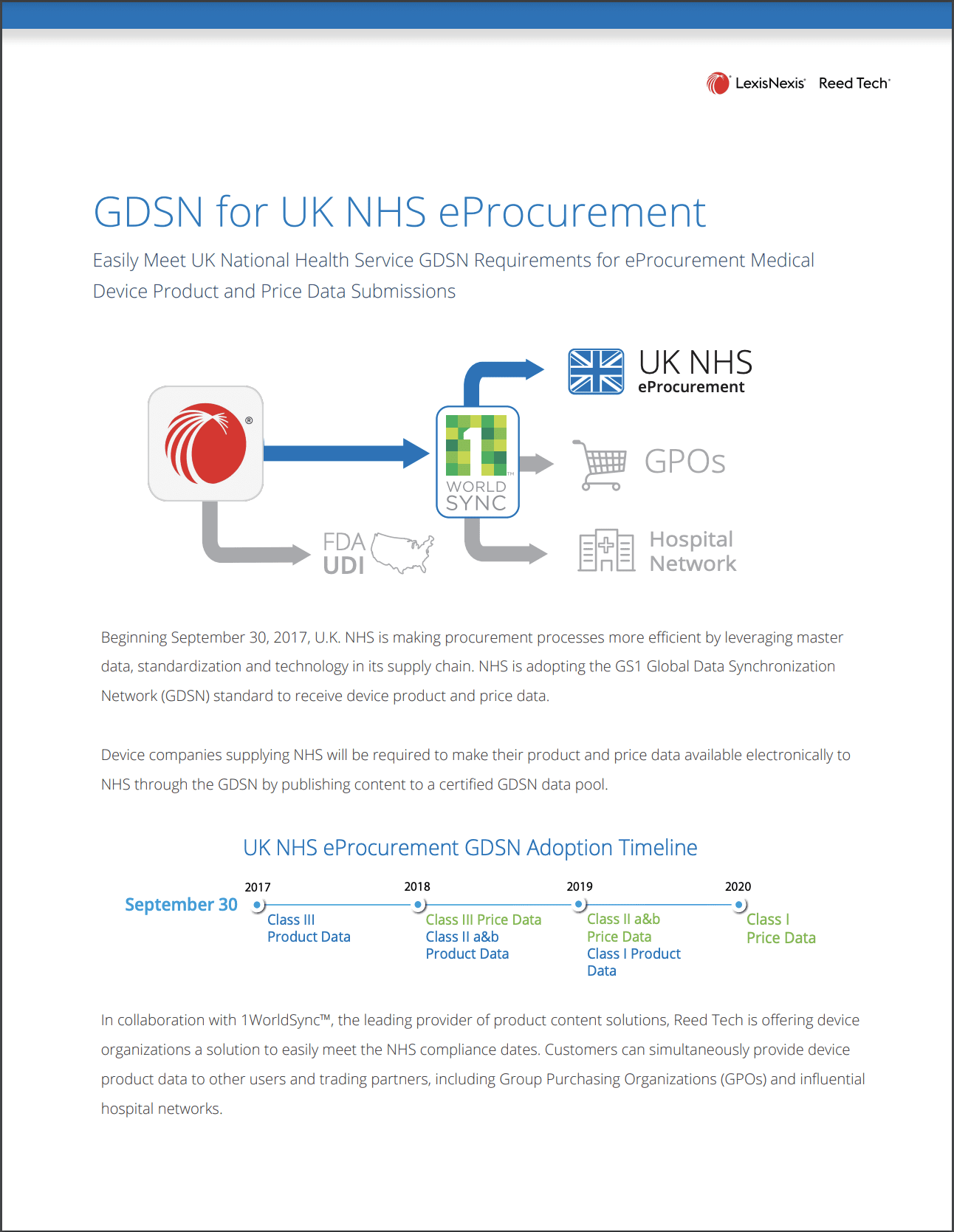
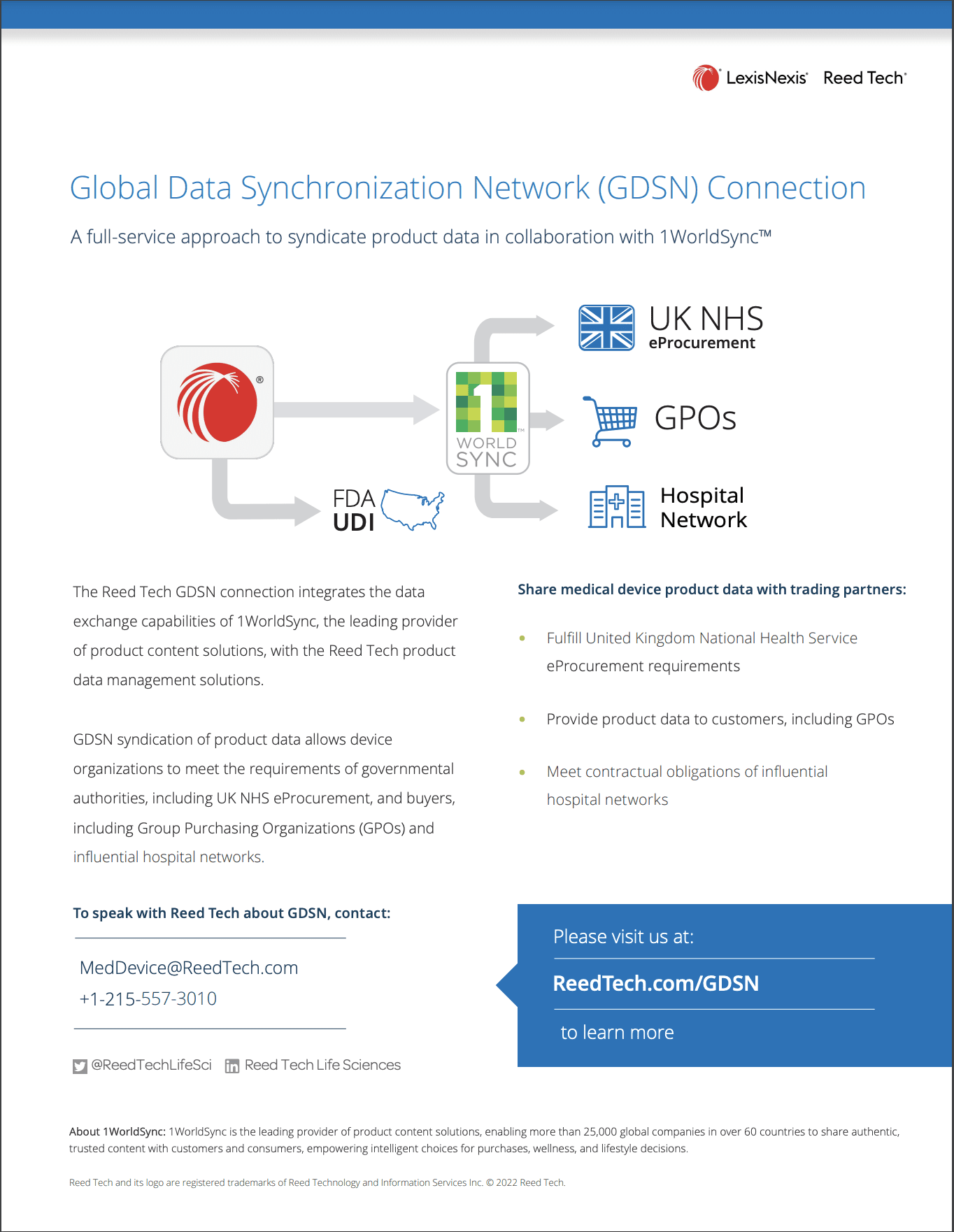
- UDI Data Solutions for Medical Devices
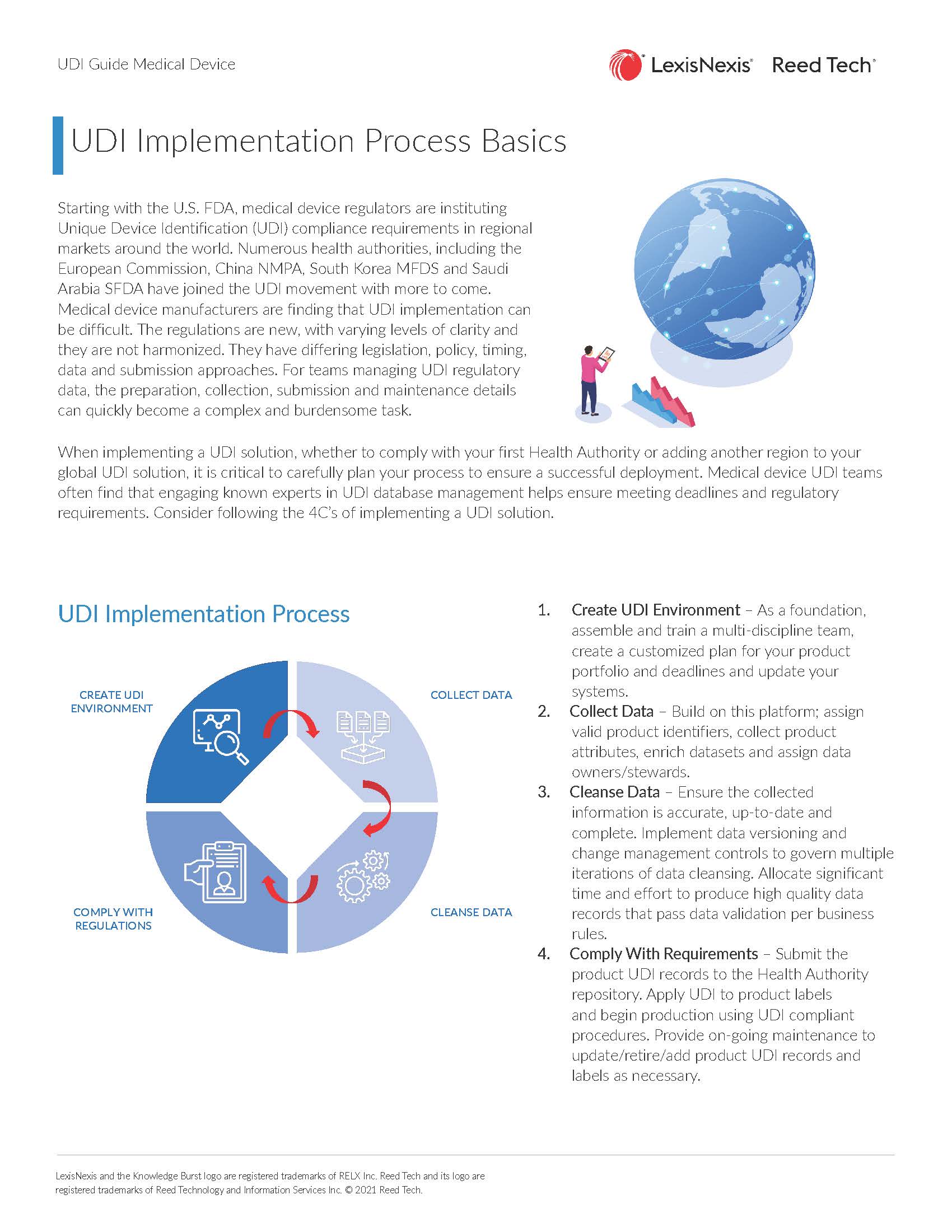
- UDI Implementation Process Basics
- Why You Need a UDI Specialist
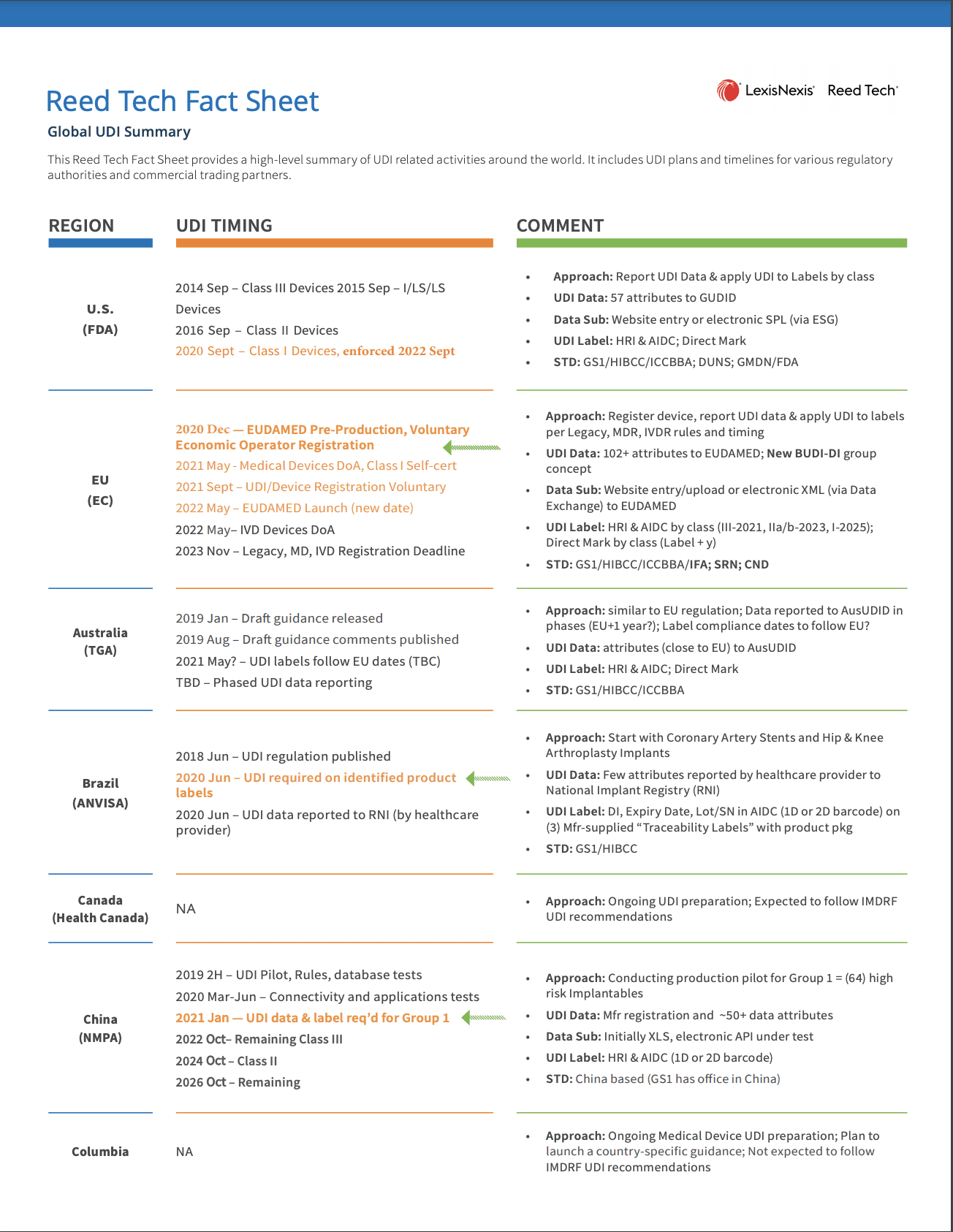
- Global UDI Summary of Health Authorities
Customer Journey for UDI Submission and Planning
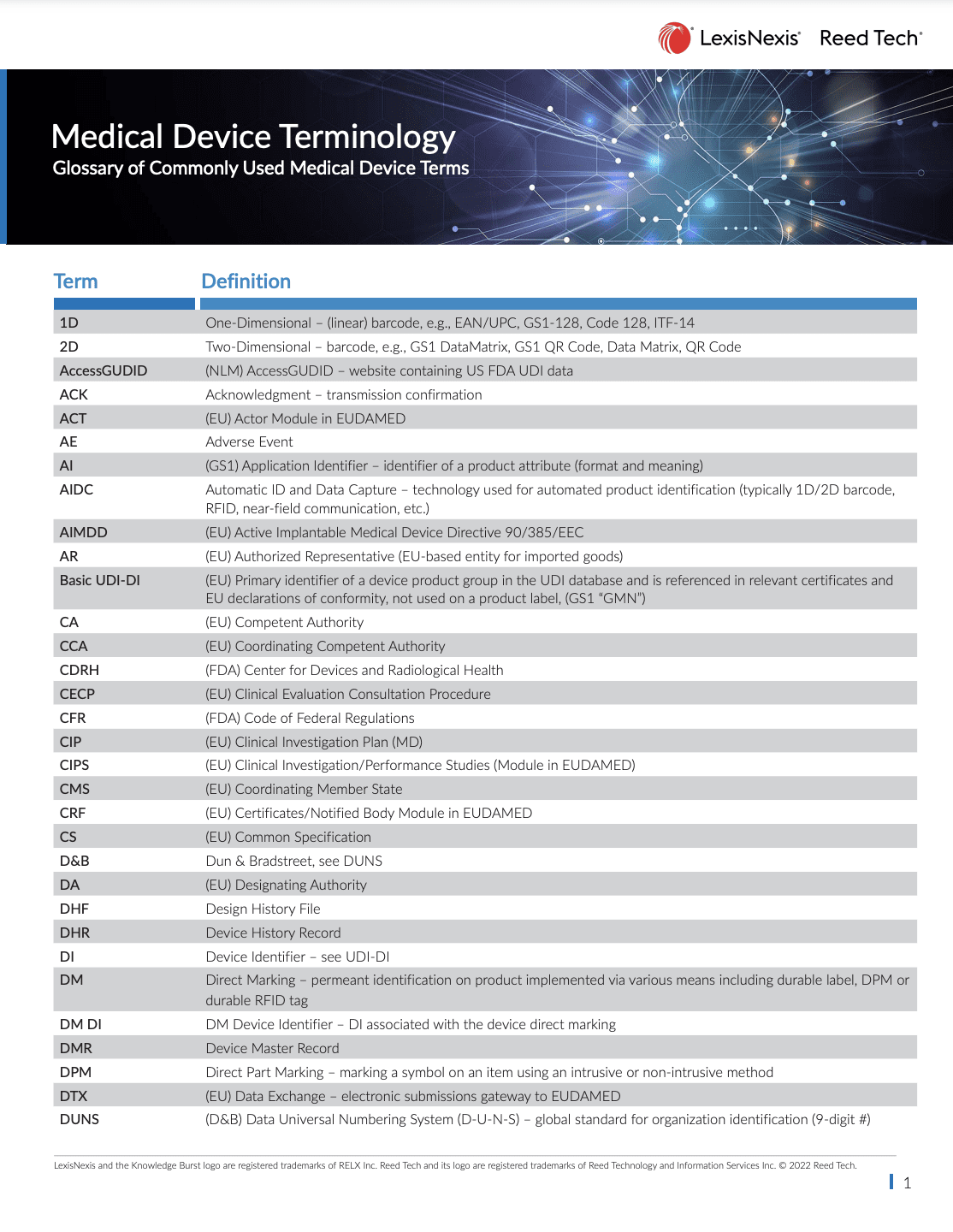
Medical Device Terminology
Alliance Partner, Greenlight Guru, Referral Link
If, for any reason, there’s potential value for customers in need of Greenlight Guru’s offerings, please send the Reed Tech referral link.
Questions? We are here to help. Visit our Knowledge Center or Contact Us.