Did you know there are multiple health authorities around the globe with current or future requirements for medical device product data specifically for Unique Device Identification (UDI) standards? The list continues to grow (US FDA, EU EUDAMED, South Korea MFDS, China NMPA, Saudi Arabia SFDA) and many others are adopting UDI. An experienced medical device labeler will tell you that there are similarities and nuanced differences from region to region. The rules and regulations are being shaped as we speak, and the impacts of those changes will inform how medical device product data is certified, registered, submitted and maintained for years to come.
Did you know there are multiple health authorities around the globe with current or future requirements for medical device product data specifically for Unique Device Identification (UDI) standards? The list continues to grow (US FDA, EU EUDAMED, South Korea MFDS, China NMPA, Saudi Arabia SFDA) and many others are adopting UDI. An experienced medical device labeler will tell you that there are similarities and nuanced differences from region to region. The rules and regulations are being shaped as we speak, and the impacts of those changes will inform how medical device product data is certified, registered, submitted and maintained for years to come.
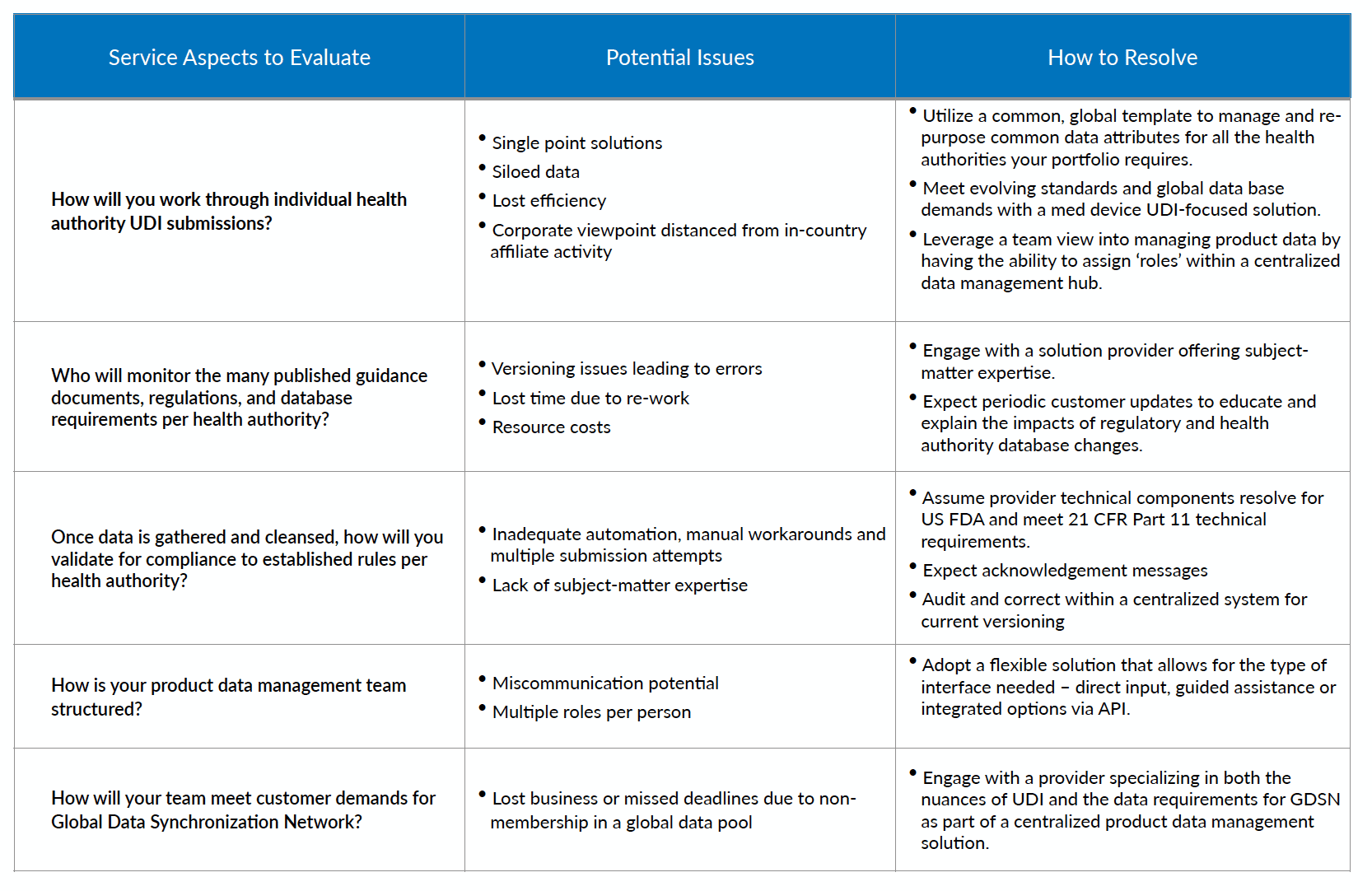
Quality data submissions will become expected standard processes for manufacturers. The benefits to industry, the supply chain and patients will feed future innovation and better outcomes in health care. With such a long-term view, managing medical device product data becomes a strategic pursuit for efficiency, accuracy and met expectations. When it comes to UDI, not all service providers are the same.
How to Evaluate & Select a UDI Specialist
Click the chart to expand:
Have questions about UDI? Email us at [email protected].