LexisNexis Life Sciences Solutions
Leverage Our Expertise
in Life Sciences
Who We Serve
LexisNexis Life Sciences Solutions serves many of the world’s top pharmaceutical labelers and medical device manufacturers. With customers from small to mid-size producers and distributors of pharmaceutical and medical device products to the very largest global leaders. Our solutions offer the means to increase productivity, comply with regulations and make more informed decisions quickly.
How We Serve
450+
Global Medical Device clients
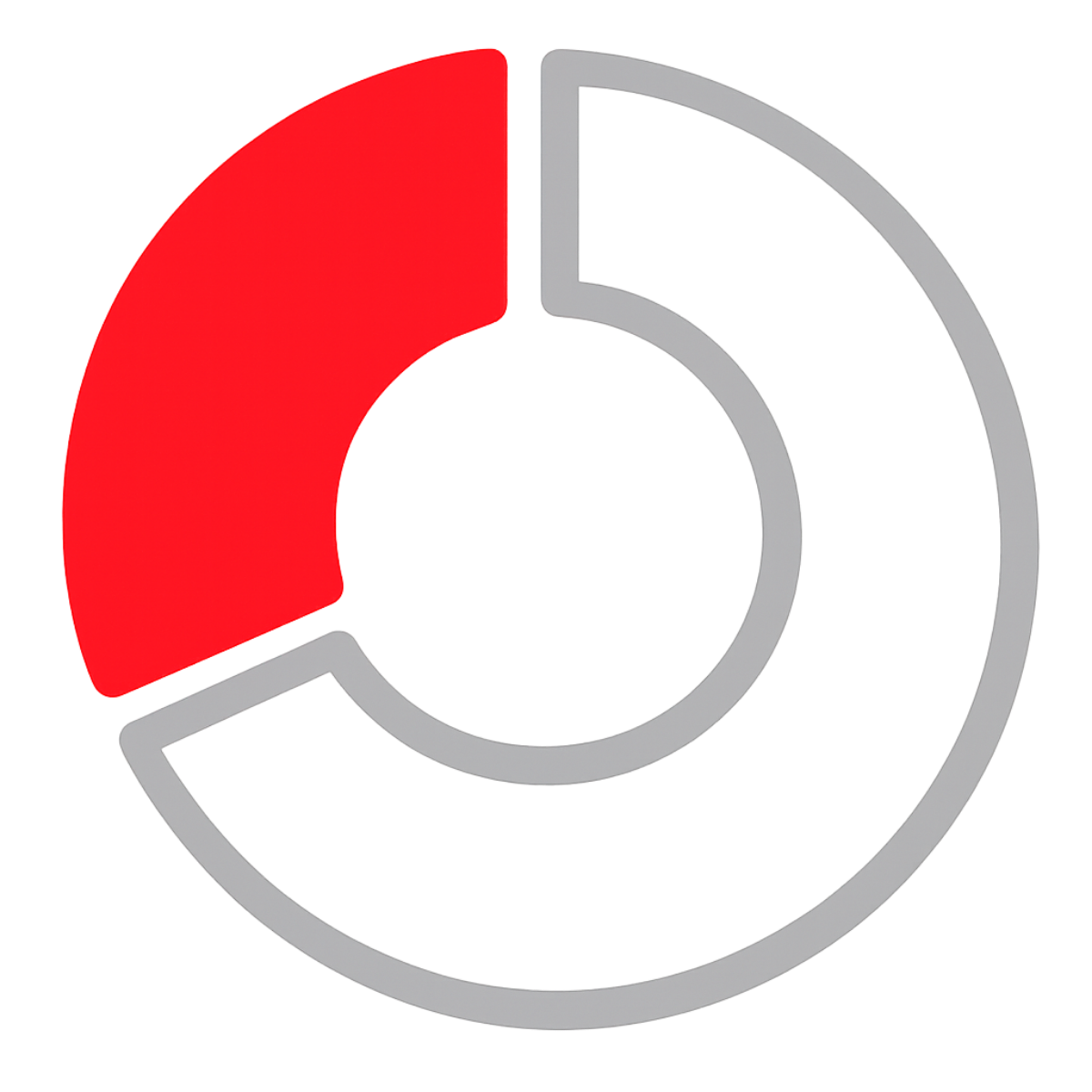
33%
Medical Device UDI records electronically submitted to US FDA GUDID
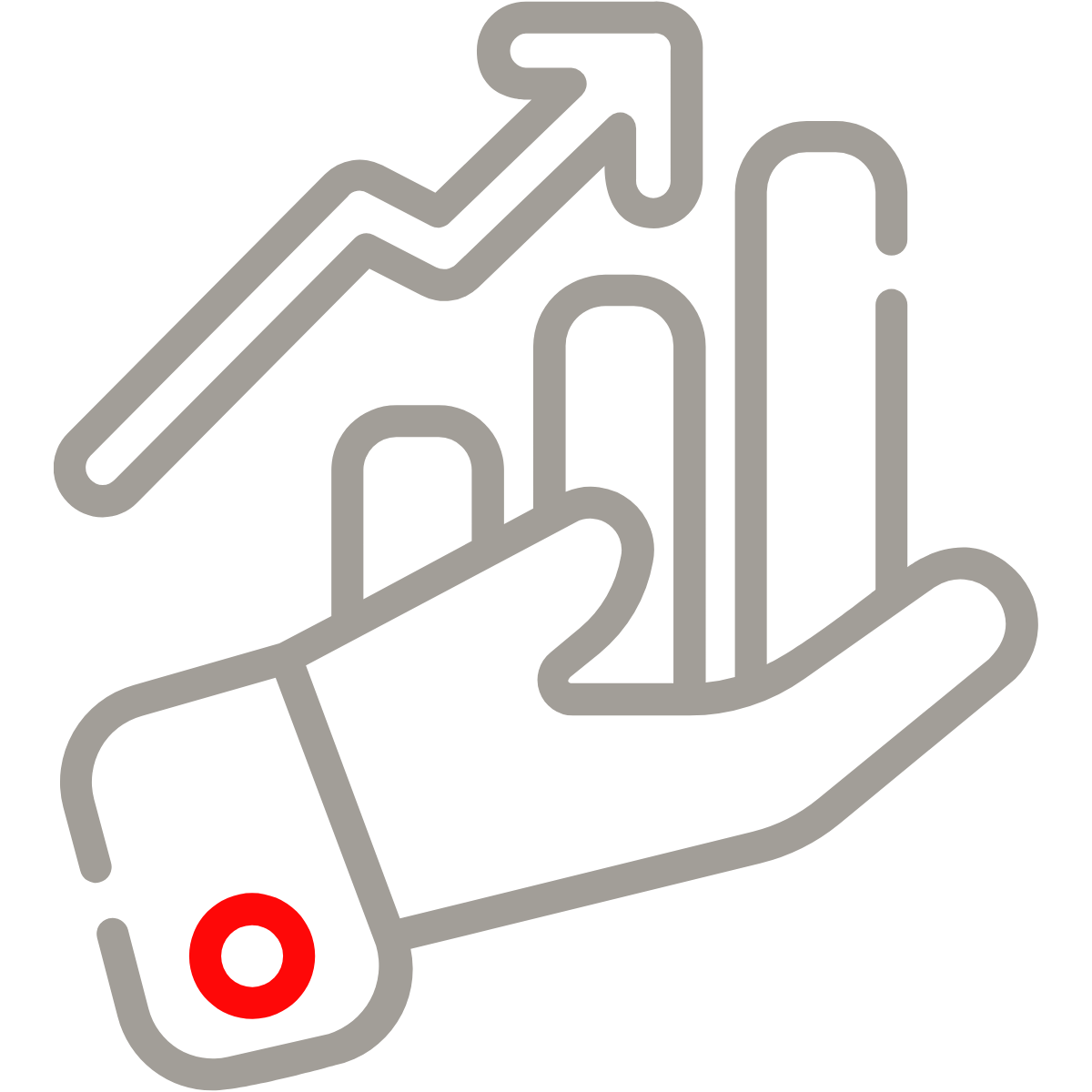
1,000,000+
Medical Device UDI records submitted globally
1,000+
Global Pharmaceutical clients
20+
Years of Pharma SPL service
99.95%
Pharma FDA SPL submissions delivered within contracted time
Webinars and Recordings
Knowledge Center
What Our Customers Are Saying
“LexisNexis Life Sciences Solutions customer service is top-notch and very friendly, helpful and professional.”
“Excellent customer and technical support. LexisNexis Life Sciences Solutions is a true partner – an extension of our internal resources. Their software solutions are enabling us to successfully meet UDI compliance dates in the US and UK.”
“LexisNexis Life Sciences Solutions has experts in Drug Listing and UDI and we can always count on them for their guidance for the best approach to be taken when needed.”
The entire LexisNexis Life Sciences Solutions team has supported our numerous clients with SPL generation. Never once have they failed us!

Affiliations