On January 23, 2024, the European Commission proposed a legislative amendment to address two major issues in the EU Medical Device Regulation (MDR) and the In Vitro Diagnostics Regulation (IVDR). We asked our in-house expert, Gary Saner, to explain the proposed changes and how they might affect medical device manufacturers. As of April 25, 2024, the EC has approved the amendment and the next step will be approval by the European Council and publication in the Official Journal of the EU is anticipated in May.
Gary Saner is a Sr. Manager of Information Solutions in the Reed Tech Life Sciences group. He is a subject matter expert on Drug Labeling, Medical Device UDI, and other structured content reporting to regulatory agencies and commercial organizations. He has over 40 years of experience in software development, process management, and data administration, with the last 19 years focused on the Life Sciences industry. With a deep understanding of regulations, business requirements, and systems, he has helped shape and implement successful solutions at Reed Tech for data management, validation, processing, and submission of Drug Labeling content, Cosmetic information, and Medical Device UDI data. He serves as chair of the industry’s Structured Product Labeling (SPL) Technical Team.

wWhen the European Union releases a new proposal, understanding its meaning can be challenging and requires careful attention. In the following text, you will find a direct quote from the new proposal (now approved) and Gary’s explanation of its high-level meaning.
European Commission:
“Firstly, it aims to further extend the transitional period for certain IVDs to mitigate the risk of shortages of these products, especially of high-risk IVDs, which are used, for example, to test for infections in blood or organ donations or for blood grouping for transfusions.
“Secondly, the proposal aims to enable a gradual roll-out of the electronic systems integrated into the European database on medical devices (‘EUDAMED’) that are finalized, instead of deferring the mandatory use of EUDAMED until the last of the six modules is completed.”
Gary’s Insights:
The approved amendment highlights that there may be shortages in the In-Vitro Diagnostic (IVD) market if no changes are made to the regulations. The reasons behind this are that most IVDs, which previously accounted for 8% but now account for approximately 80% under the IVDR regulations, require IVDR conformity assessments by Notified Bodies. However, only 12 IVDR Notified Bodies are available, and these conformity assessments can last for an average of 18 months. Moreover, they often involve new requirements for the Notified Bodies and the manufacturers, making the process slow.
To encourage the adoption of IVDR by manufacturers, the transition period extension for placing Legacy IVDs on the market is offered only to those manufacturers who demonstrate significant progress towards IVDR implementation, such as installing an IVDR-compliant Quality Management System (QMS) by May 26, 2025, and engaging an IVDR Notified Body by specific deadlines.
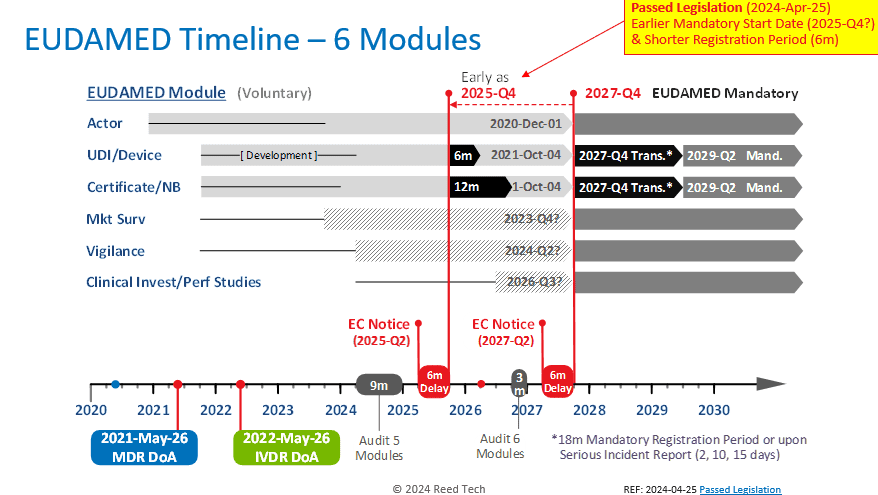
Various Member States and other stakeholders encouraged the EC to mandate individual functional EUDAMED modules before all six modules are fully functional. Three modules, Actor, UDI/Devices, and Certificates/NBs, have been open for voluntary production use for multiple years. Two additional modules, Market Surveillance, and Vigilance, are expected to be completed by 2024-Q2.
The estimated completion date for the Clinical Investigations/Performance Studies module is not earlier than Q3 2026. Considering an audit and a 6-month implementation delay, all six modules won’t be required before Q4 2027.
The approved amendment aims to empower the EC to mandate the usage of a module that has been successfully audited and declared functional without waiting for all six modules to be fully functional. This change will facilitate the European Commission’s plan to start using functional modules as early as 2025-Q4, two years earlier than the current plan and just a year and nine months away. In addition, once the UDI/Device mandatory registration period starts, the current 18-month transition period is proposed to be collapsed to only a 6-month transition period.
The approved MDR/IVDR amendment was passed by the Council and Parliament with only minor modifications and no significant delay. Now that the regulations are passed, the EC is expected to publish an updated timeline indicating their plan to mandate various EUDAMED modules. (expected May 2024)
Please refer to the graphic below for a clear understanding of the new timeline.
Questions about health authorities and Unique Device Identification (UDI)? We monitor health authorities around the globe for the latest requirements and exceptions. If you have UDI questions, we can help. Email us: [email protected] or call +1-215-557-3010
