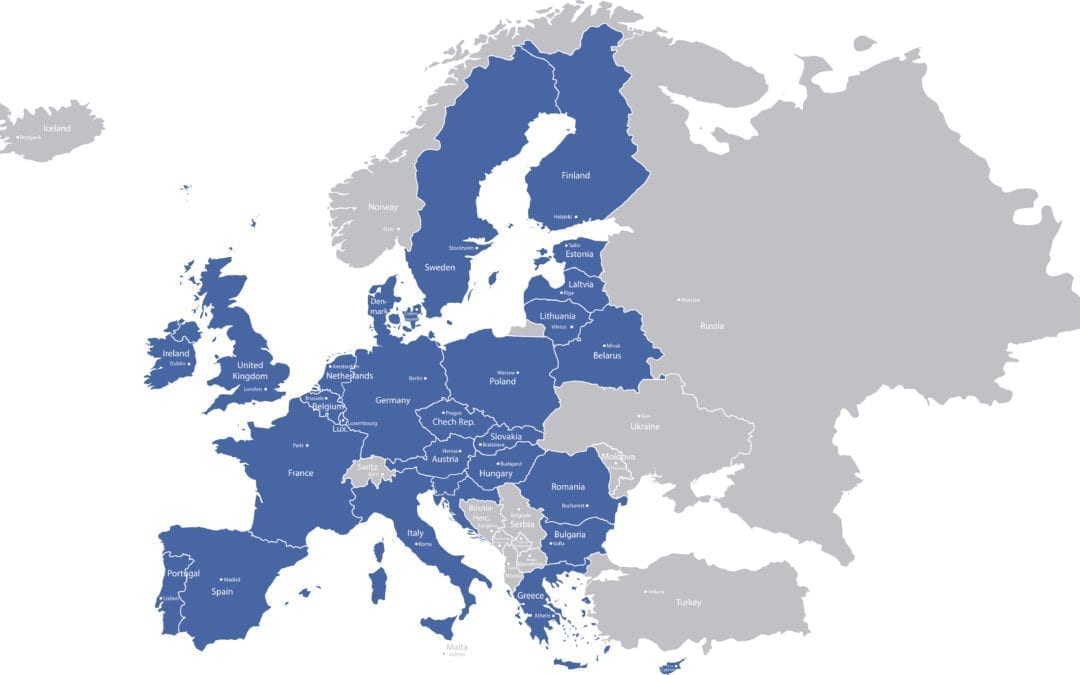
With the EUDAMED go-live date rescheduled multiple times, manufacturers face conflicting strategies of when to make UDI/Device registration submissions to EUDAMED.
Explore our library of blogs, short videos, virtual event recordings and training topics

With the EUDAMED go-live date rescheduled multiple times, manufacturers face conflicting strategies of when to make UDI/Device registration submissions to EUDAMED.

On January 23, 2024, the European Commission proposed a legislative amendment to address two major issues in the EU Medical Device Regulation (MDR) and the In Vitro Diagnostics Regulation (IVDR).

Quick Insights from our experts on the EC Proposal concerning the IVDR transition and proposed MDR/IVDR amendment, possibly affecting the EUDAMED rollout. These are short segments created from our latest presentation, prepared exclusively for UDI customers.
Start the year with clean data and a firm understanding of what you must do for EUDAMED UDI compliance in 2024. UDI compliance readiness can be confusing. To help, we’ve gathered top tips from expert Gary Saner regarding upcoming milestones in the EUDAMED roadmap.
The EUDAMED timeline has experienced several delays and revisions since its inception, primarily due to the complexity and magnitude of the project.
Explore the significance of the AusUDID rollout, its objectives, and how the TGA requirements for UDI differ from FDA regulations.
EUDAMED UDI Compliance is a critical aspect of the European Medical Device Regulation (MDR) that requires manufacturers to adopt a standardized UDI system for better traceability and safety of medical devices within the EU market. The compliance process may seem...

The Food and Drug Administration (FDA) has implemented the Unique Device Identification (UDI) system to enhance patient safety, improve post-market surveillance, and facilitate the identification of medical devices. The UDI system requires manufacturers to assign a...

What are the potential consequences for medical device manufacturers not following through with product data submissions and labeling requirements? In a recent inspection, violations were identified regarding a manufacturer’s Unique Device Identification (UDI)...

Courtesy notification of news from FDA: The U.S. Food and Drug Administration (FDA) recently initiated two initiatives to improve the completeness and quality of the Global Unique Device Identification Database (GUDID). As a medical device manufacturer or other...