The European Union (EU) requires manufacturers to supply ‘implant cards’ for patients with implanted medical devices, as prescribed in Article 18 of Regulation (EU) 2017/745 on medical devices. The requirement’s purpose is to give patients easy access to important information about their implants. Providers or medical institutions are to deliver the implant cards to patients, but device manufacturers will be responsible for producing the cards with specific formatting, information, and language requirements.
The wallet-sized cards, unique to the implanted medical device, are designed to support patient safety in three ways:
- Through precise information: Patients can identify the specific implant in their bodies, including name, serial and lot number, and thus more easily access information about a device using EUDAMED, the European database on medical devices, and other websites.
- Through convenient identification: Patients can show they have special care needs, such as at airport security checkpoints.
- During emergency care: The cards can inform first responders and emergency clinicians of a patient’s implanted device in the event of a medical emergency.
Guidelines on Patient Implant Cards
Guidelines from the Medical Device Coordination Group (MDCG) of the EU provides a non-exhaustive list of medical devices that fall under the regulation for implant cards. The list of more than 85 devices includes pacemakers, lumbar shunts, intraocular lenses, coronary stents, breast implants, and gastric bands. Exempted from the regulation are “simple” implants such as dental fillings, tooth crowns, pins, and sutures.
According to MDCG guidance, the implant cards must be the size of a credit card, ATM card, or ID card. Text, including numbers, letters, and symbols, must be at least two millimeters high and written so it can be easily understood by a layperson and in the language determined by the relevant member state.
The card should list the following information about the implant:
-
-
- Device name
- Device type
- Unique Device Identification (UDI). The UDI should be in automatic identification and data capture format, such as linear or 2D-barcodes, and the UDI device identifier (UDI-DI) should be in human-readable format.
- Serial number or, where applicable, lot or batch number
- Name and address of the manufacturer
- Website of the manufacturer
-
The card also must include blank fields for patient identification information, which will be filled out by the implanting provider or healthcare institution. This information gives:
-
-
- Patient name or ID
- Implantation date
- Name/address of the healthcare institution where the implantation was performed
-
With multiple nations affected by the implant card requirement, the MDCG advises manufacturers to use approved symbols to provide a common “shorthand” for all stakeholders and avoid national versions of the cards. A guide to the symbols and instructions for healthcare professionals on how to fill out the implant card should be included in shipments to providers and healthcare institutions. It is recommended that manufacturers provide this information in a leaflet written in the language required by the member state. Manufacturers are also advised to update any relevant implant card information via a patient-accessible website listed on the card.
Example: Suggested design of a foldable Implant Card
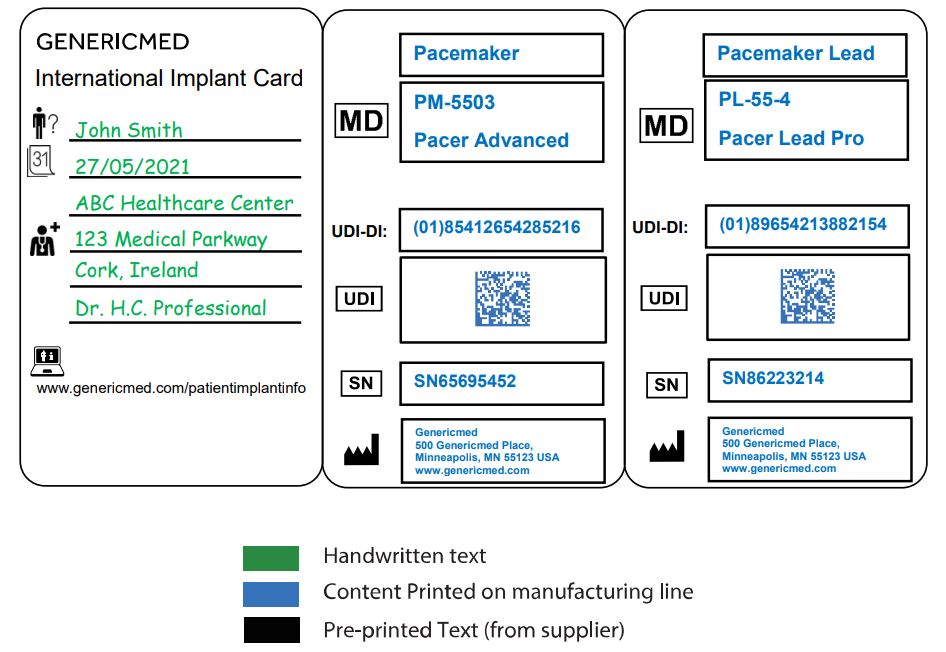
Now that the MDR DoA (Date of Application) has passed, per Article 18 of the MDR, manufacturers of some implantable devices are obligated to provide a patient implant card as EUDAMED becomes fully available. The IVDR Date of Application is 26 May 2022.
As guidance continues to develop for UDI and device registration for MDR/IVDR, Reed Tech stands ready to navigate the complexity for medical device manufacturers and other stakeholders. To learn more, email us: [email protected]
Sources:
ec.europa.eu, MDG 2021-11 Guidance on Implant Card – Device types (May 2021)
