The Australia Therapeutic Goods Administration (TGA) has announced several updates to the go-forward plan for implementing UDI compliance
News of note:
• TGA has announced that they will have a similar model to the FDA, adopting some elements of the EUDAMED model
• A third consultation is planned for the end of this year
• Voluntary compliance to begin January 2023
The Australia TGA website has posted a number of updates to help inform the public of their process. See the convenient links below to learn more.
You may wish to subscribe to the TGA email list or participate in the upcoming ‘Sandpit’ UDI pilot. Reed Tech would be happy to join you as a technical partner if you wish to participate.
Legislation/Regulation
- 2019-Jan-07 Australian TGA released proposal for UDI System
- 2019-Apr-04 TGA UDI Action Plan
- 2020-Sep-23 TGA opened UDI Consultation Survey
- 2020-Oct-06 Gov’t approved $7.7 million for TGA AusUDID
- 2021-Feb-19 Therapeutic Goods Amendment (2020 Measures No. 2) Bill 2020, created AusUDID
- 2022-Aug-31 Consultation #3
- 2023-Jan TBD Regulations & Guidance
TGA UDI Information (materials)
- 2021-Jul-20 TGA UDI Webinar #2
- 2021-Aug-21 TGA UDI Webinar #3
- 2021-Sep-21 TGA UDI Webinar #4
- 2021-Oct-19 TGA UDI Webinar #5
- 2021-Nov-16 TGA UDI Webinar #6
- 2022-Mar-22 TGA UDI Webinar#7
- 2022-Apr-19 TGA UDI Webinar#8
- 2022-May-17 TGA UDI Webinar #9
- 2022-Jun-21 TGA UDI Webinar #10
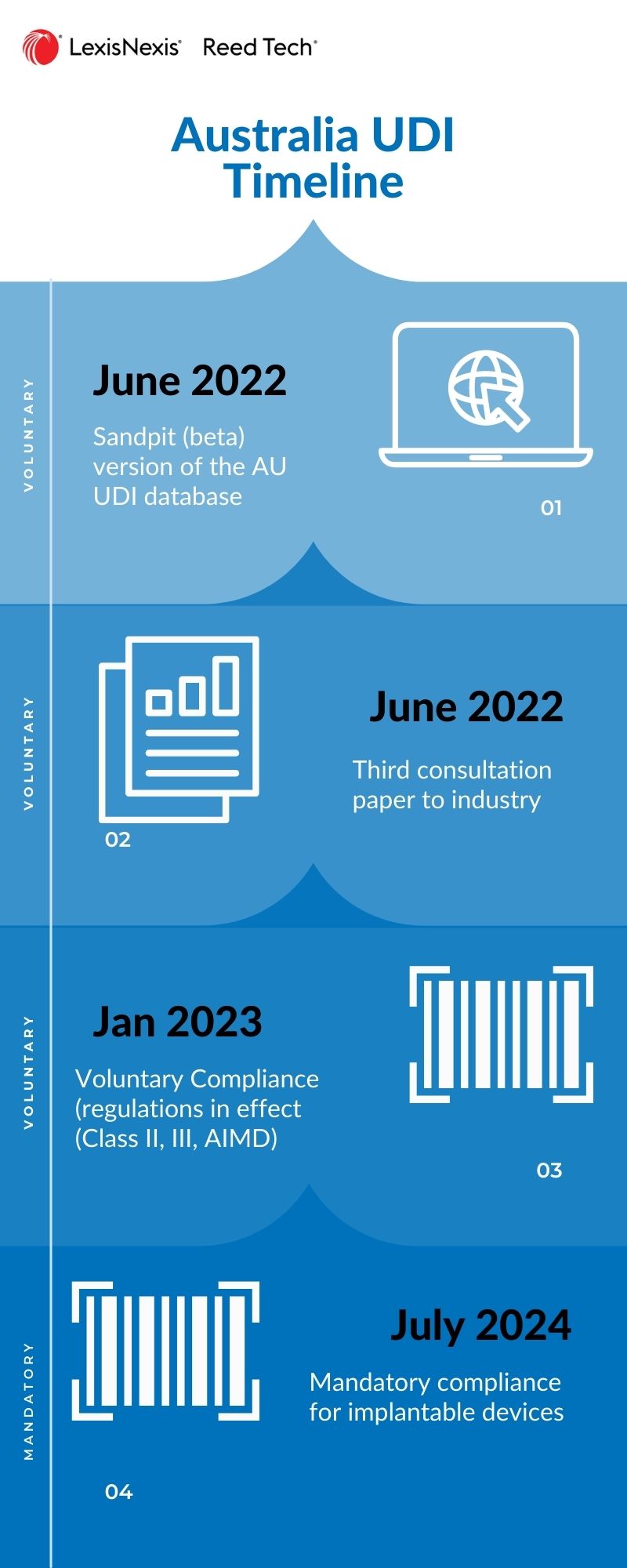
UDI Timing
- 2022-Jul-04 AusUDID Sandpit (test, general use)
- 2023-Jan Vol. Compliance (high risk II, III, AIMD)
- 2024-Jul Required Compliance (Implants, UDI labeling, UDI data)
TGA UDI Information (website)
- Medical device reforms: Establishment of a UDI system; Overview, links
- TGA Unique Device Identification system (UDI home page): Benefits, Progress, News
- TGA UDI Action Plan Progress (webpage): History, Progress, Communication, Timing
- TGA UDI Communication (webpage): Forums, Webinars
- Subscribe to TGA email list
- TGA UDI Pilot
- Queensland is planning UDI pilot – contact TGA UDI team at [email protected]
Listen to this quick 6-minute update, recorded July 2022:
We will continue to monitor Australia TGA progress for UDI and provide updates. Contact Reed Tech to see how product data management can be handled effectively and efficiently through flexible team roles and data validation tools in a dedicated solution such as SingleSource™.
Email us: [email protected]