What is Basic UDI-DI?
Unique to the European Commission and EUDAMED, Basic UDI-DI is a product group identifier for related medical devices, like a ‘family’ of devices. The relationship is child to parent. Multiples of devices may be grouped under a family group or Basic UDI-DI. Each medical device UDI-DI has only one Basic UDI parent. All medical devices included in the Basic UDI-DI group share the same intended purpose, risk class, essential design, manufacturing characteristics, and certification. All medical devices included in the Basic UDI-DI group have the same model/group data attributes reported to the EUDAMED UDI/Device module.
The European Commission Medical Device Coordination Group (MDCG) format has specific requirements, including a maximum amount of 25 characters and the inclusion of a check digit or character. Basic UDI-DI is created by the manufacturer and issued by a medical device standards group (GS1, HIBCC, ICCBBA, IFA).
Those devices complying with MDR/IVDR regulations will have a Basic UDI-DI. Note that there is not a grouping concept for ‘legacy devices’. For EUDAMED legacy devices, the EUDAMED DI is created and is a 1:1 relationship.
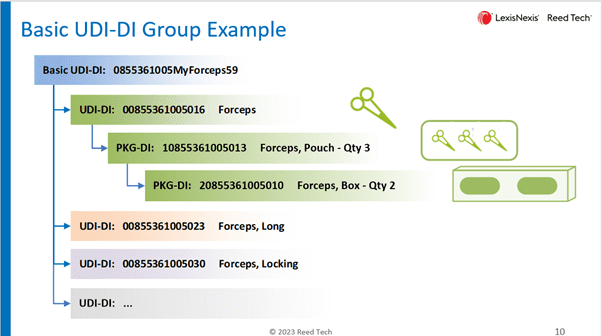
Here is an example of a Basic UDI-DI grouping highlighting forceps.
Why is Basic UDI-DI Important?
Basic UDI-DI is used throughout the lifecycle of a product in the EUDAMED database. Basic UDI-DI is not used in any labeling, physical marking or GS1 AIDC data carrier (like a bar code) on associated trade items. Specific use cases include the following.
Conformity Assessments: If a device needs a Conformity Assessment (before being placed on the market) as well as EU Technical Documentation Assessment Certificate, EU Type-examination Certificate and EU Product Verification Certificates may be involved.
Manufacturer Documentation: For the EU Declaration of Conformity, Technical Documentation and Summary of Safety and Clinical Performance (if the device is a Class III or implantable device), Basic UDI-DI would be included.
Data Reporting to EUDAMED: Device Registration and submission of UDI Core Data Elements in the UDI/Device Module.
Export Devices: Basic UDI-DI is included in Certificate of Free Sale to the member state.
For a quick introduction to the concept of EUDAMED Basic UDI-DI, see this short video.
See an example of EUDAMED Basic UDI-DI implemented per a GS1 standard in this short video.
