Understanding EUDAMED
EUDAMED is an online system that aims to centralize and harmonize the data related to medical devices across the European Union. It is a vast repository containing information on medical devices, manufacturers, clinical investigations, market surveillance, and other critical data. The overarching goal of EUDAMED is to strengthen regulatory oversight and patient safety within the EU.
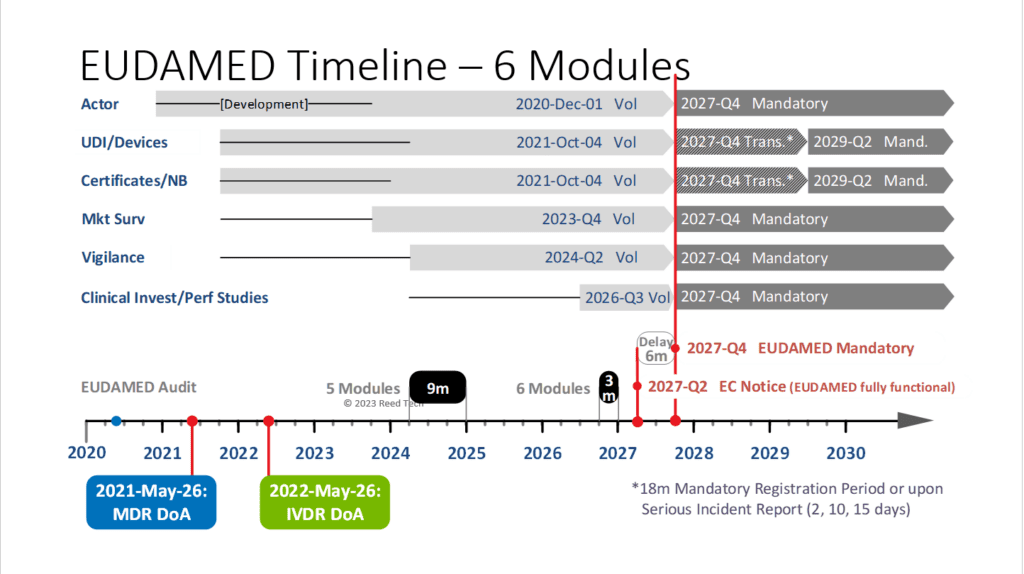
Recent Updates to the EUDAMED Timeline
The EUDAMED timeline has experienced several delays and revisions since its inception, primarily due to the complexity and magnitude of the project. The following are the most recent updates to the EUDAMED timeline:
The European Commission is evaluating the development roadmap for EUDAMED. New information became public on October 20, 2023. Development and module interdependencies are affecting the timing, according to MedTech Europe.
What is driving this?
The development of the CIPS module is taking longer than expected due to its complexity and change requests. As a result, DG SANTE has redirected resources from the ‘Use Case’ development. Therefore, the European Commission has decided to pause the development of the CIPS module until all other modules are completed.
Once the audit starts on the first five modules, they will resume working on the CIPS module development, which is expected to take over two years to complete. It is important to note that EUDAMED can only be enforced as a mandatory system once it is finished with all modules.
What does this mean for medical device manufacturers?
MedTech Europe will examine these developments in more detail and communicate their potential impact on manufacturers. The industry must closely monitor the effect of delayed transparency, continuous compliance with national databases, and legacy device registration.
Industry insiders recommend continued diligence and voluntary data submission.
Manufacturers should continue data cleansing, assigning Basic UDI-DI, and preparing for risks.
Draft new timeline:
Keep Preparing for EUDAMED submissions:
- Now: Continue preparing for EUDAMED voluntary and production environment submissions to the UDI/Device module. Use Reed Tech to create, cleanse, and store Basic UDI-DI identifiers and attributes
- 2024-Jan-23: Reed Tech has gained connection rights to submit machine-to-machine records to the EUDAMED production environment.
- 2024-May-26: Manufacturer must have Basic UDI-DI defined before engaging a Notified Body for MDR/IVDR evaluation
- 2024-Q2: 5 EUDAMED modules (Actor, UDI/Device, Certificates, Market Surveillance, Vigilance) finished development updates and placed into audit
- 2024-Q2+: Reed Tech recommends start submitting a few and up to full inventory of UDI records into voluntary UDI/Device Playground module (EUDAMED 5 modules enter audit)
- Register Early:
- EU Member States and the industry in general may prefer devices to use voluntary EUDAMED production modules
- Other Health Authorities may prefer devices to use voluntary EUDAMED production modules
- 2026-Q4+: Reed Tech recommends submitting a few UDI records into voluntary UDI/Devices Production module (EUDAMED system enters full audit). Continue monitoring all regulatory health authorities for updates.
* The timeline has not yet been officially finalized. We will continue to monitor any changes as they develop.
Additional Updates on New Proposals
As of 2024-Jan-23, The EU Commission proposes to extend transition periods for certain IVDs, gradual roll-out of EUDAMED and an information obligation in case of interruptions to supplies.
See the latest postings by The EU Commission:
Questions about health authorities and Unique Device Identification (UDI)? We monitor health authorities around the globe for the latest requirements and exceptions. If you have UDI questions, we can help. Email us: [email protected] or call +1-215-557-3010
