With the US Class I UDI enforcement quickly approaching, you may be wondering “what about older inventory”?
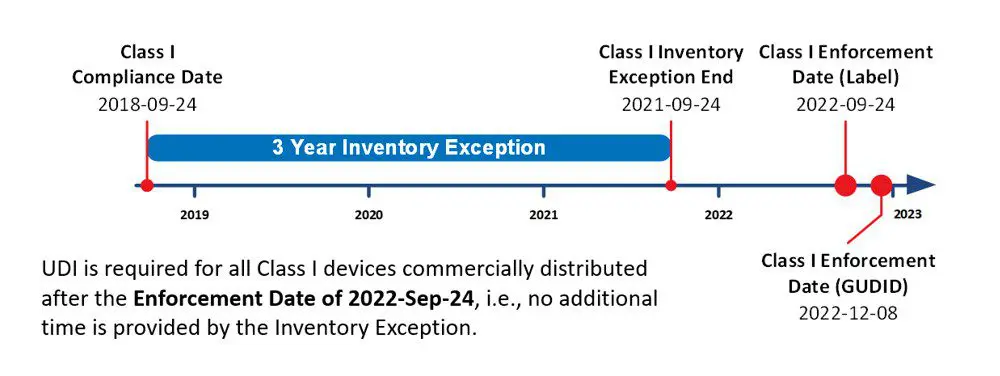
In 2013, the FDA granted a UDI exception for 3 years after the corresponding Compliance Date for finished medical devices manufactured and labeled without Unique Device Identification (UDI) as of the Compliance Date, commonly referred to as the 3-Year Inventory UDI Exception or the ‘Final Rule.’ Such devices meeting the criteria could be commercially distributed for 3 years after the corresponding Compliance Date without complying with UDI requirements. At the end of the 3-year period, any undistributed inventory would need to be reprocessed to comply with current UDI requirements, i.e., UDI Label and GUDID reporting.
In general, the UDI final rule requires device labelers (typically, the manufacturer) to:
- Include a Unique Device Identifier (UDI), issued under an FDA-accredited issuing agency’s UDI system, on-device labels, device packages, and in some instances, directly on the device.
- Submit device information to the Global Unique Device Identification Database (GUDID).
The Final Guidance from FDA concerning Class I devices allows exemptions for many products. Exemptions include ‘Good Manufacturing Practice’ (GMP), if the product is carrying a UPC label currently, is 510(k)-exempt and sold exclusively to consumers over-the-counter. Other scenarios require compliance.
It is a common misconception that the Class I three-year inventory exception applies to UDI products starting September 24, 2022. This exception period ended September 24, 2021. No additional time has been extended. Products in inventory must be in compliance by the September enforcement date. Now that the date of compliance has passed it is important that you ensure completion of the regulatory directives as soon as possible.
LexisNexis Reed Tech can assist your organization in understanding the impact of US FDA and other global health authority UDI compliance requirements. Our UDI solution is uniquely positioned to provide you the training and support needed throughout the data collection, submission and maintenance process, saving you time and money along the way.
Global organizations complying with US FDA UDI early will benefit from flattening the UDI workload. Apply the lessons learned from the well-defined US UDI mandate. An established UDI process and proven data management system are valuable assets in meeting other regional health authority UDI regulations in 2022 and in the future.
Reed Tech is here to assist you in complying with the US, EU, China, South Korea and other regional UDI requirements. Email us at: [email protected] or visit us at www.ReedTech.com/UDI.
For more information about UDI Inventory Compliance, please view this short video featuring subject matter expert Gary Saner.
Note: Video below was recorded from a recent webinar on Class I UDI submission requirements and exceptions. View the full webinar here.
