For medical device manufacturers and distributors, UDI product data submission to Health Authorities/Regulators is a multi-step process with rules and goals, much like a ‘team sport’.
My local affiliate (Authorized Representative) handles UDI in that region, how can we all have visibility into a single version of our UDI data?
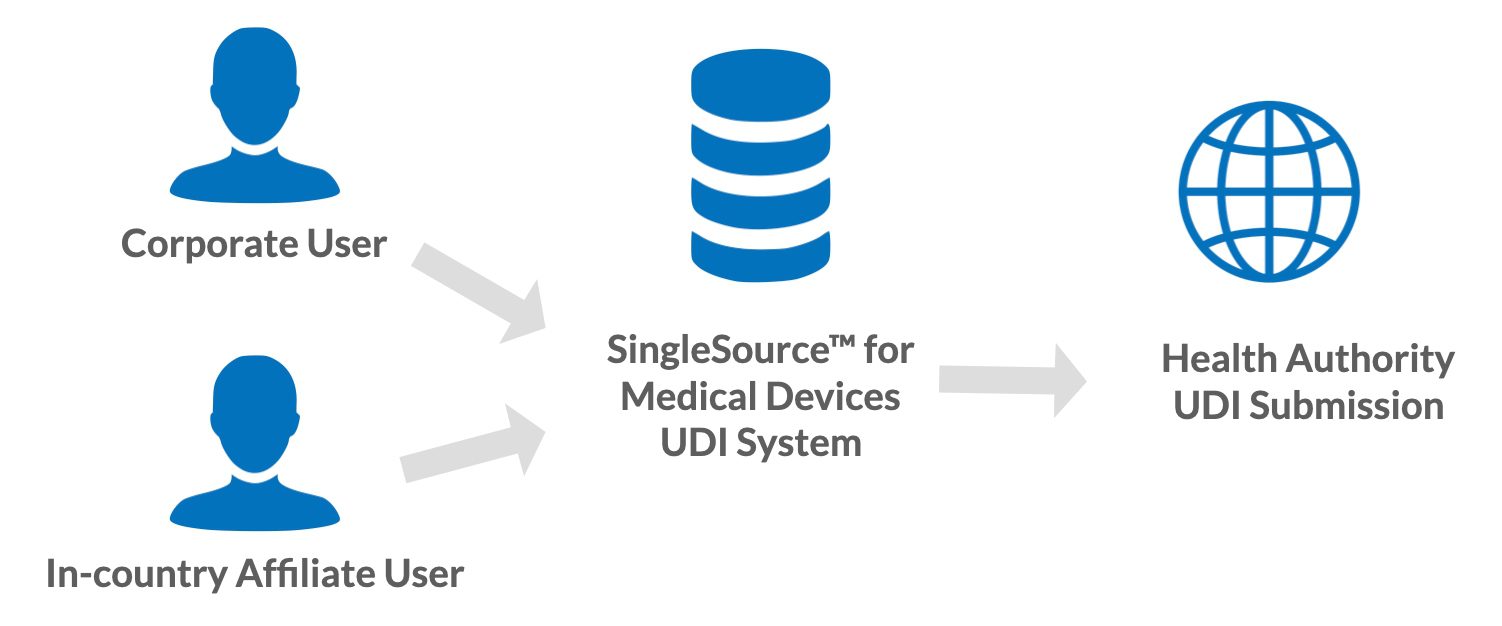
A local user/affiliate can be assigned a role within the Reed Tech SingleSource™ for Medical Devices system for active involvement in the data workflow. Usually the local user has expertise and language translation of the content, providing valuable assistance. With an assigned role inside of SingleSource™, a local user could populate and upload translated UDI data via the Reed Tech file to the appropriate worksite within the system. Depending on the scenario, control and visibility can be shared between the corporate user and the local user. This dynamic provides a centralized view into the data with transparency for both the corporate office and the in-country representative. A ‘team’ view streamlines visibility for better communications concerning data and the opportunity to export UDI data into internal systems if needed.
Do you need an Authorized Representative in European countries?
To obtain a CE certification and manage risk under the European Commission MDR/IVDR (Medical Device Regulation and In Vitro Diagnostic Regulation), an Authorized Representative must be engaged in the applicable country to represent your company. This position may be called an EC REP or AR with the primary responsibilities of ensuring device registrations are submitted, acting as the custodian of the Technical Files, the CE Declaration of Conformity and making files available if requested for inspection. They may also be called upon to assist with Incident and Field Safety Corrective Action (FSCA) activity, as a liaison for your company and your distributors. Often, an Authorized Representative is an independent entity that has regulatory affairs expertise, usually located in said country. The AR may be a single person or an agency specializing regulatory obligations and requirements. Regulatory expertise is needed to respond accurately when occasion arises with Competent Authorities (Health Authority/Regulator or Ministry of Health representing a country or region.) In most cases, the AR activity is seen as an extension of quality and risk management activity.
If an AR is not engaged, complications will likely arise for importing products into the country. Your Notified Body will require an AR before issuing a CE certificate. Packaging on the labeling, outside of the product and Instructions for Use (IFUs) will require the AR name and address. Engaging with an Authorized Representative is seen as a long-term commitment. If an AR change is needed, labeling costs to accommodate the change could be impactful to a budget.
What about Brexit?
For companies EU and non-EU based, a UK-based Authorized Representative (UK Responsible Person) is needed to continue selling medical devices into the UK. In-country representation is required.
Is an Authorized Representative required by other Health Authorities?
Yes, for any medical device manufacturer, a legal entity or Authorized Representative is required by all health authorities to serve as a regulatory liaison between the medical device company and the Health Authority. In most regions, the associated tasks are about the same: ensuring device registration, coordinating any changes for submission, supporting adverse event reports, recalls or FSCA, and acting on behalf of the manufacturer when inquiries of a regulatory nature arise. A distributor can in some cases be an AR, however expertise in regulatory requirements and in-country nuances around this activity is needed to avoid risk.
What are some of the larger Health Authorities actively developing or requiring UDI submissions currently?
- US FDA GUDID
- European Union (EU EUDAMED)
- China NMPA
- South Korea MFDS
- Netherlands Dutch National Implant Registry (LIR)
- Saudi Arabia (SFDA) and others
Reed Tech provides experienced guidance for UDI submissions to global health authorities. With SingleSource™ for Medical Devices, UDI data is managed throughout the product lifecycle in a compliant, cloud-based SaaS environment and includes scalability for additional volume and global health authorities. Companies can include any designated Authorized Representatives as ‘users’ on their behalf within SingleSource™. Assign roles wherever your team is located and securely manage UDI record submission and product data lifecycle management directly via the user interface. To learn more about how Reed Tech can assist with UDI, contact us: [email protected]
Reed Tech does not offer prescribed Authorized Representative support services.
