EUDAMED UDI Compliance is a critical aspect of the European Medical Device Regulation (MDR) that requires manufacturers to adopt a standardized UDI system for better traceability and safety of medical devices within the EU market. The compliance process may seem daunting, but its significance cannot be underestimated.
By starting the compliance process now, medical device manufacturers can stay ahead of deadlines, avoid bottlenecks, gain a competitive advantage, and prepare for post-market surveillance efficiently. The early implementation also allows manufacturers to identify and resolve potential challenges and adapt supply chain processes.
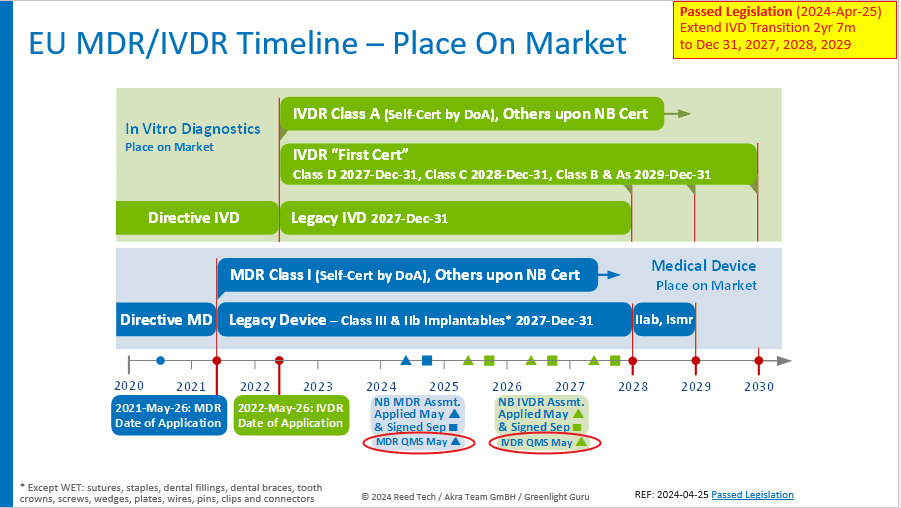
Proactive adherence to EUDAMED UDI Compliance ensures regulatory compliance and reflects a commitment to patient safety and responsible business practices. Embracing this change will pave the way for a more transparent, efficient, and secure medical device industry in the European Union. The most recent update to the EU MDR/IVDR Timeline is shown below.
Reed Tech provides valuable support in preparing for UDI submissions to global health authorities.
Contact the Reed Tech Team (or call 1-215-557-3010 / email [email protected]) for more information and explore how Reed Tech can help you meet your global Medical Device UDI data management challenges and requirements.
