 The Food and Drug Administration (FDA) has informed industry that the February 15, 2022 date for submitting 2020 data per Reporting Amount of Listed Drugs and Biological Products Under Section 510(j)(3) of the Federal Food, Drug, and Cosmetic Act, originally referred to as a deadline, will now be the recommended date.
The Food and Drug Administration (FDA) has informed industry that the February 15, 2022 date for submitting 2020 data per Reporting Amount of Listed Drugs and Biological Products Under Section 510(j)(3) of the Federal Food, Drug, and Cosmetic Act, originally referred to as a deadline, will now be the recommended date.
This means the reporting date is non-binding and not a requirement. This update is because comments to the draft guidance are still being considered, including recommended updates to the reporting timeframes.
As 2021 is quickly coming to a close, the US Food and Drug Administration (FDA) is instituting a new annual distribution report for all listed drugs. According to the FDA, “Each registrant that lists a drug must report to FDA annually on the amount of such drug that it manufactured, prepared, propagated, compounded or processed (including repacking and relabeling) for commercial distribution.” This data will need to be submitted in a comma-separated file or manually entered and identified by national drug codes (NDC) tied to establishment registration (RE) and include the correct single business operation.
What Drugs are In-Scope?
The scope of this reporting will be vast, including human and animal drug products, prescription and over-the-counter (OTC) monograph drugs, finished dosage form products, active pharmaceutical ingredient (API), medical gases, homeopathic products and other types of listed drugs. There are a very limited amount of exceptions including products or categories thereof exempted by an order under section 510(j)(3)(B). Currently the FDA has proposed two biological exceptions: a) blood and blood components for transfusion, and b) cell and gene therapy products, where one lot treats a single patient.
What Distribution Data is Reported?
Product distribution quantities are reported for the calendar year (January 1st through December 31st) for each registered establishment (ER) identified with the associated primary business operation. Along with general submitter information, the report includes detailed distribution counts by month for the outermost and innermost (if appropriate) packaging level identified by National Drug Codes (NDC).
When Must Distribution Data Be Reported?
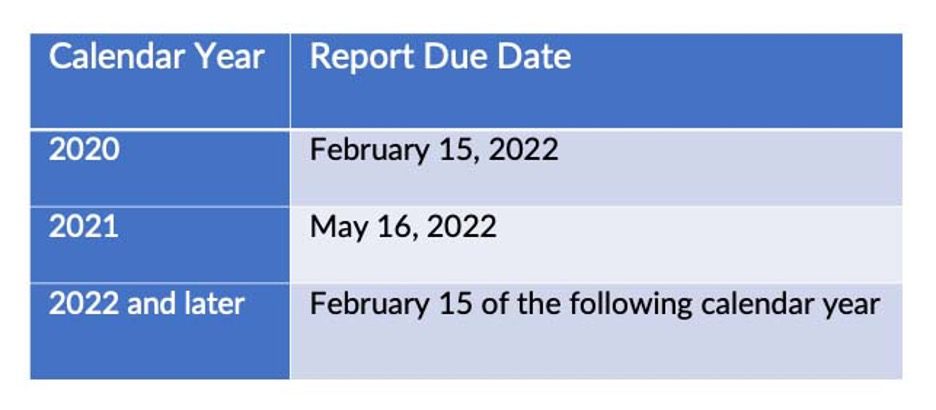
The Drug Distribution Report will be added to annual reporting requirements and must include distribution counts for the calendar year starting January 1st through December 31st for the applicable year. Once the standard schedule takes effect in 2023, this will be the previous calendar year and will be due in February. The reporting schedule for the implementation period is suggested but not required.
Uniquely, this draft guidance, along with a final technical conformance guide, was proposed in October 2021 with a 60-day comment period. The comments on the draft guidance are currently under review, including suggested timeframes. It is still a good idea to launch your Drug Distribution Report response: identify your team, learn the requirements, establish processing and submission procedures, locate the distribution data sources, and begin collecting/aggregating data as soon as possible.
Background
This new reporting requirement was brought about by the enactment of the CARES act in March 2020. The CARES act provided authority to have more visibility into drug company supply chains to identify and mitigate potential drug shortages. According to FDA, “section 3112(e) of the CARES Act added new section 510(j)(3) of the FD&C Act, which requires that each person (including repackers and relabelers) who registers with FDA under section 510 of the FD&C Act with regard to a drug must report to FDA annually on the amount of each listed drug that was manufactured, prepared, propagated, compounded, or processed by such person for commercial distribution.”
For more information about this new annual requirement or how Reed Tech can help with your data management and submission, contact us at [email protected] or +1 (215) 557-3010.
