Each year pharmaceutical companies, including both manufacturers and private label distributors, are subject to numerous guidances and mandates to develop and maintain compliance with the FDA. Though every company is different and each situation is unique, the majority of these deadlines fall in the final quarter of each year and many require structured product labeling submissions. Reed Tech customers often have questions about which annual requirements they are required to comply with and how and when to do so. To shed light on this topic for our customers and industry colleagues, Reed Tech subject-matter experts Gary Saner and David Wilson recently presented a webinar Achieving Compliance: Pharma Annual Deadlines.
Overview of FDA Annual Requirements
FDA drug requirements fall into five categories: marketing authorization, establishment registration, product listing, labeling and records and reporting. Over the better part of the last 2 decades, FDA has been migrating the submissions for these requirements into Structured Product Labeling (SPL) format in accordance with Health Level 7 (HL7) standards. Manufacturers and distributors need to be preparing for the 2022 deadline for submitting REMS (Risk Evaluation and Mitigation Strategy) in SPL. Not every company is the same, but typical annual SPL submission timing is as follows:
-
-
- Biologic Lot Distribution Data Report (LDR) – every 6 months
- Drug Sample Distribution Report (DSD) – annually by April 1 for previous year
- Generic Drug Facility Self ID (SID) – annually in May for upcoming year
- Establishment Registration (ER) – update upon change; no changes= Q4 submission
- Drug Labeling/Listing (DL) – update upon change; review and update in June and December
- Drug Listing Blanket No-Change (BNCC) – Q4 submission
-
In addition to FDA, Health Canada is in the process of capturing product monograph (PM) data in structured XML format. Industry is currently in phase II – voluntary product submissions, as the timeline for mandatory phases grows shorter, it is likely annual submission deadlines will be applied.
Year-End Requirements and Reporting
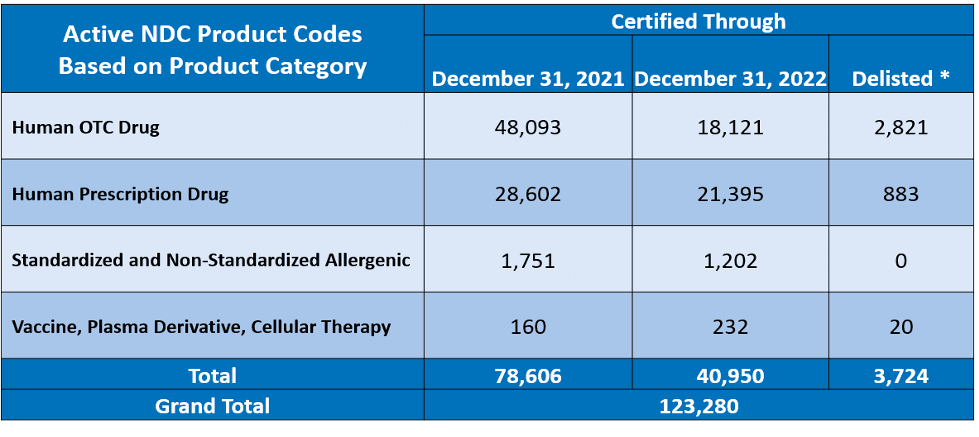
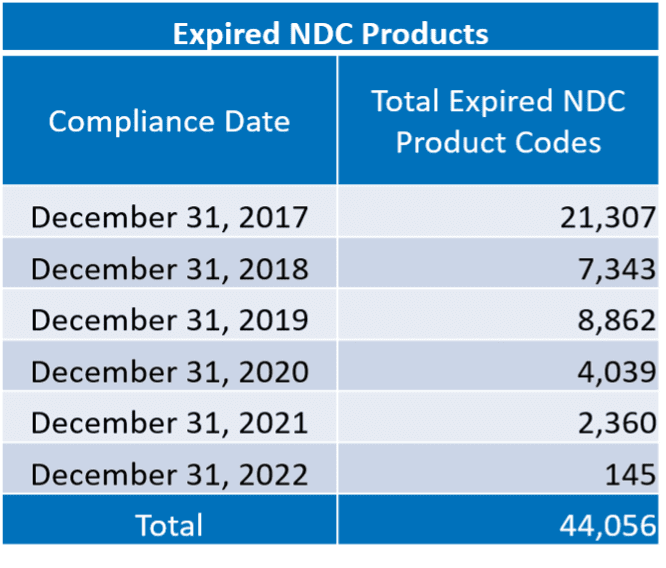
FDA provides industry report cards showing if products are active or expired. Simply put, products that do not meet the annual deadline requirements have an expired status. Please note an expired status is not the equivalent of delisting a product. These report cards are available to the public via FDA’s website. Below is a breakdown of the current report cards conducted by Reed Tech.
Structured Product Labeling Requirements
Data Storage and Management
Industry faces many challenges in finding efficient and compliant means to store and manage data. The data often needs to be shared among colleagues while maintaining version control and version history. As such, there are many benefits to using a Drug Label Data Management Tool such as SingleSource™ for Drug Products.
SingleSource™ for Drug Products is a web-based application developed to 21 CFR Part 11 compliant standards that allows drug product manufacturers and distributors to collect and store their global product listings, establishment facility and labeler company data in an easy-to-use master submission data library. It allows users to centrally manage FDA human prescription, over-the-counter and animal drug product submissions data in Structured Product Labeling (SPL) format. To learn more about SingleSource™ for Drug Products, contact [email protected].
View the full webinar here!

