Reed Tech subject matter experts, Gary Saner, Information Solutions Senior Manager and Patti Shragher, Medical Device Senior Account Executive and Team Lead, host a webinar discussion dedicated to Unique Device Identification (UDI) requirements for health authorities in Asia. Gary and Patti also explore the regulatory regions, address frequently asked questions and discuss what your team can do now to prepare for product data submissions in Asia. A few key takeaways are noted below.
UDI Timelines for Asian Health Authorities
China National Medical Products Administration (NMPA) UDI issue date occurred on August 23, 2019 with UDI rules consistent with the International Medical Device Regulators Forum (IMDRF) UDI guidance, however no full schedule has been published.
- A UDI pilot established Class III devices (high-risk implants and instruments with 69 categories and 51 data attributes) to be in the Batch 1 devices, which occurred on January 1, 2021, requiring UDI data and label.
- Remaining Class III devices, not covered in Batch 1, are considered Batch 2 and go into effect on June 1, 2022.
- NMPA has not yet provided official notice of the UDI Compliance Dates for Class II and Class I devices.
- The webinar discusses further information for timing, data submissions, UDI manufacturing date, registration and translation.
South Korea Ministry of Food and Drug Safety (MFDS) required Class IV, Class III and Class II UDI data (40 data attributes total) to be on labels and reported to the Integrated Medical Device Information System (IMDIS).
- Class I medical device UDI data must be reported by July 1, 2022.
- MFDS is still accepting Class IV, III and II medical device submissions to the IMDIS database.
- We encourage you to submit as soon as possible if you haven’t yet already.
- The presentation also discusses a Track and Trace program in South Korea and its associated timeline.
UDI timelines for other regions (including India, Japan, Saudi Arabia, Singapore, Taiwan, Turkey, UAE, Vietnam) are noted in the webinar’s appendix.
Global UDI Landscape, Trends and Challenges
While this webinar focuses on health authorities in Asia, manufacturers may have multiple global health authorities in their markets, plus downstream customers. Timelines for product data submission and labels vary per health authority. In addition, there are numerous UDI technical challenges and, sometimes when applicable, varying issuing agencies per health authority. It becomes a challenge to harmonize data among these health authority channels. There is a growing need for manufacturers to consider a single, flexible centralized UDI data management approach.
Gary and Patti also note who is responsible for UDI data submission for China NMPA and South Korea MFDS, as both health authorities require an in-country local entity to perform the UDI and Product registration.
Reed Tech SingleSource™ Solution Overview
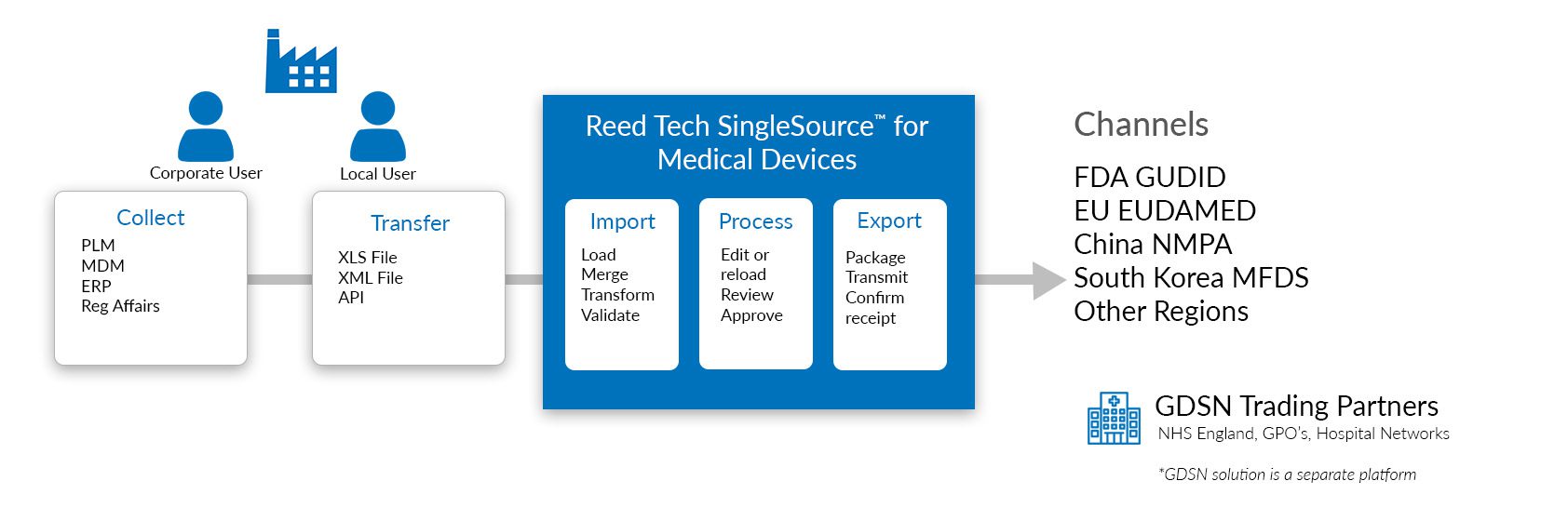
Reed Tech SingleSource™ allows both local and corporate users to work within the system to meet regional UDI submission requirements. In the webinar poll, attendees preferred the electronic machine-to-machine product data (UDI) submission method when available.
What to Do Now
-
-
- Create UDI Environment: Create UDI governance team, identify UDI requirements for your products, evaluate your situation, create UDI plan, prepare UDI infrastructure, establish UDI solution
- Collect Data: Setup product ID standard, assign identifiers to your portfolio, assemble UDI data, establish data governance
- Cleanse Data: Verify data, normalize and validate source UDI data, establish version and approval controls
- Comply with Regulations: Create regulatory accounts (for Asia, this will involve a local representative), submit UDI data, verify submission success, maintain data and systems
-
Additional Resources
Reed Tech provides valuable support with questions you may have about global health authority requirements, such as Asia, UDI data attributes and timelines. Contact us today at [email protected] or 1-215-557-3010.
