Author: John Lorenc, Sr. Manager Regulatory Solutions, Reed Tech
Fiction:
Most global health authorities subscribe to a GDSN data pool, therefore, UDI compliance can be achieved by a standard GDSN data pool publication.
Fact:
One of the largest health authorities, the US FDA does not subscribe to a GDSN data pool. Registration for US FDA GUDID is a separate activity that may be added as a service between a GDSN data pool and a medical device manufacturer. Typically the service includes data mapping and transformations from the GDSN standard to the submission standard required by the health authority, for example HL7 SPL for the US FDA.
Registration with a Health Authority should be considered the first formal submission process. Submitting to a GDSN-certified data pool for commercial publication of the data should come next.
As more Health Authorities begin publishing requirements, we find subtle differences region to region. These differences may not always translate to supply chain requirements.
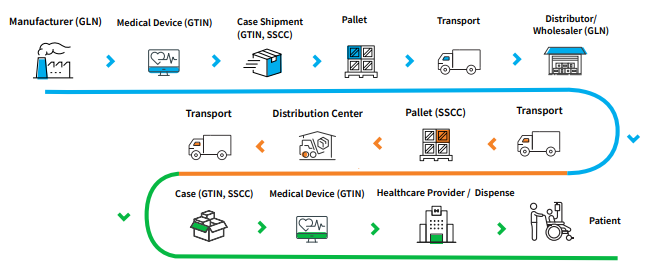
For instance, the EU regulations have introduced a new identifier ‘Basic UDI-DI or BUDI-DI’ as an assignment requirement for device submissions to EUDAMED. The BUDI-DI data attributes group similar features and quantity packaging. However, from a supply chain perspective, the Basic UDI-DI is not part of the product labeling or packaging. It is considered a regulatory identifier required for the medical device Declaration of Conformity (DOC), Summary of Safety and Clinical Performance (SSCP) and other documentation. As a result, the GDSN standard may require an update, typically as part of a Healthcare release, to accommodate for the new attribution introduced via each regional UDI initiative. It is important to note that product data accuracy and completeness rests solely with the manufacturer. Engaging with an experienced compliance solutions provider is important for ensuring deadlines are met. For an idea of how the GDSN works and what a basic plan for assigning GTINs and submitting attributes may entail, see the summary reference doc below:
Questions about medical device related topics such as supply chain, GTINs, or Unique Device Identification (UDI)?
Contact us, we’d be happy to assist! Email: [email protected] or call +1-215-557-3010.