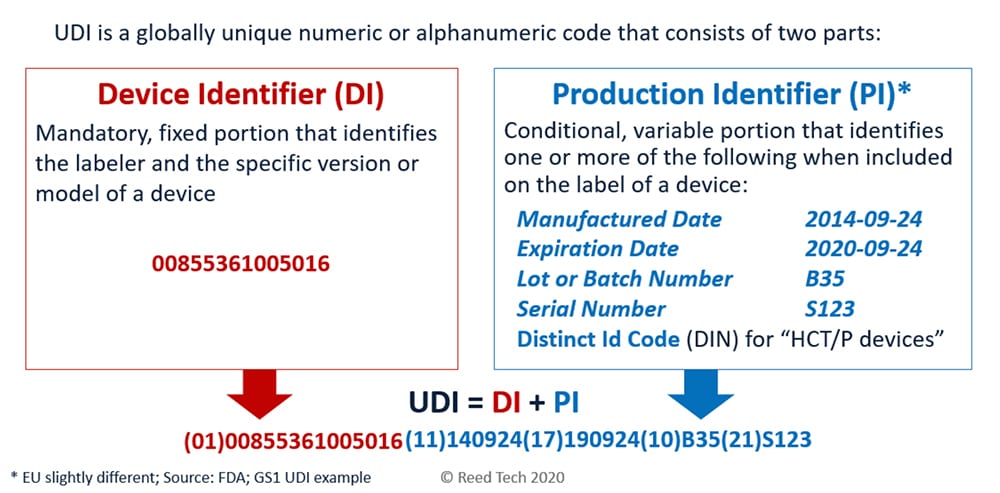
Unique Device Identification (UDI) labeling remains a hot topic among medical device manufacturers under pressure to comply with UDI requirements for US FDA GUDID and other emerging health authorities around the globe. EU EUDAMED is underway and China’s NMPA, South Korea and others have published guidelines. UDI is becoming integrated into medical device labeling all over the world. Medical device manufacturers doing business globally will encounter growing regulatory complexity, while also ensuring quality, streamlined processes and cost control.
Here are some UDI best practices and explanations that will help ensure quality and compliance as you work to make your labeling UDI compliant.
What is UDI labeling?
UDI is medical device identification comprised of a Device Identifier (DI) and Production Identifier (PI) per an approved Issuing Agency that allows the unambiguous identification of a specific device on the global market.
Common mistake: too many barcodes on a label
If the label contains multiple barcodes, the medical staff may not know which one to scan. Barcodes can contain multiple pieces of data separated with application identifiers. The software that decodes the scanner reading can parse the data, eliminating the need for multiple barcodes.
Best practice: Concatenate all data into a single barcode using application identifiers.
Should you use linear or 2D barcodes?
Both have advantages and disadvantages. Linear barcodes are more universal and can also be read by older and cheaper barcode scanners. The major disadvantage is the length of the barcode and the space it requires on the label.
2D barcodes can hold much more content in much less space but cannot be read by old linear laser scanners. This is less of an issue with time, since 2D scanners are increasingly affordable and widely available. Hospitals tell us that when they buy scanners, their preference is 2D.
Best practice: Use the linear Code 128 barcode type if space permits, but make sure it meets the size requirements. If you are unsure whether it meets these requirements or you have very limited space on the label, use the two dimensional DataMatrix code. Either way, be sure to check the barcode quality with verification equipment or with your issuing agency. They may require you to pay a small fee for the verification.
Source: GS1
Deciphering human-readable information
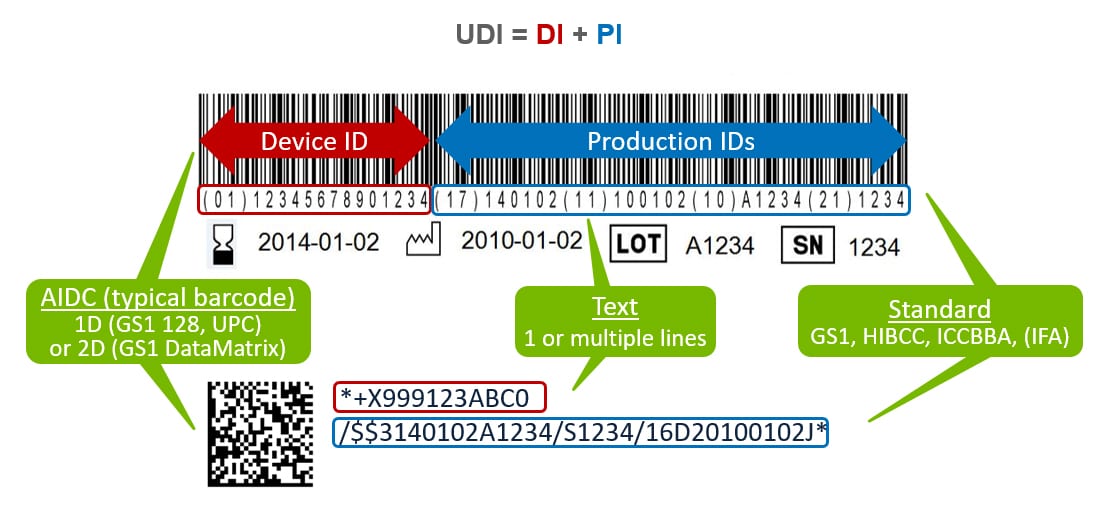
The FDA specification requires the UDI to appear in both machine-readable and human-readable form.
While there is usually human-readable content below the barcode, this is not enough to be UDI compliant. This text is meant to be used if the barcode cannot be decoded by the scanner. In this case, the user would key in the barcode content manually.
Best Practice: UDI requires device identification and production identification data to be printed separately and clearly. This is because the medical staff that applies the device will not know the application identifiers under the barcode. The lot number and expiration date need to be presented very clearly in a specific format on the label so the medical staff can quickly identify the data for patient safety.
Navigating the complexities of regulatory requirements for UDI can quickly become a burden. Get in touch with the expertise at Reed Tech for help. email us: [email protected]