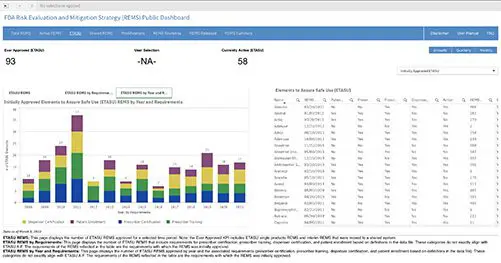
As of December 28, 2022, all new REMS (Risk Evaluation and Mitigation Strategy) submissions and all REMS updates will be required to be submitted in SPL (Structured Product Labeling) format per the FDA. This is in compliance with the Final Guidance on REMS issued by...