Knowledge Center
Explore our library of blogs, short videos, virtual event recordings and training topics
Recent Blogs

What’s Been Happening with Health Canada XML PM Mandates?
Reed Tech recently presented a webinar to update customers with the most recent information about XML product...

DIA Global Labeling Takeaways: Patient Materials and Health Literacy
Reed Tech habitually attends industry conferences and forums to share our expertise and gain knowledge from our...
FDA Drug Distribution Amounts Report: Frequently Asked Questions and More
In late 2021, the US Food and Drug Administration (FDA) is instited a new annual distribution report, Reporting Amount...
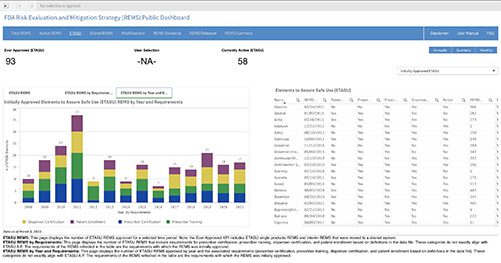
Public Dashboard Now Available for REMS Data
A new public dashboard has been launched by the Food and Drug Administration (FDA) for public access to data for drugs...

Updated Deadline: FDA Announces New Annual Drug Distribution Reporting Requirement
The Food and Drug Administration (FDA) has informed industry that the February 15, 2022 date for submitting 2020 data...

FDA Withdraws 216 ANDAs
Abbreviated new drug applications (ANDAs) are subject to complying with requirements of FDA annual reporting—often the...

Overview of Pharma Annual Deadlines and Requirements
Each year pharmaceutical companies, including both manufacturers and private label distributors, are subject to...

Proposed Changes to OTC Sunscreen GRASE and Labeling Regulations
The FDA has proposed new changes to the regulations regarding over-the-counter sunscreen products. Perhaps the most...

New Drug Application (NDA): Back to the Basics
What is a New Drug Application (NDA)?Every new drug in the United States needs to come to market through a New Drug...
