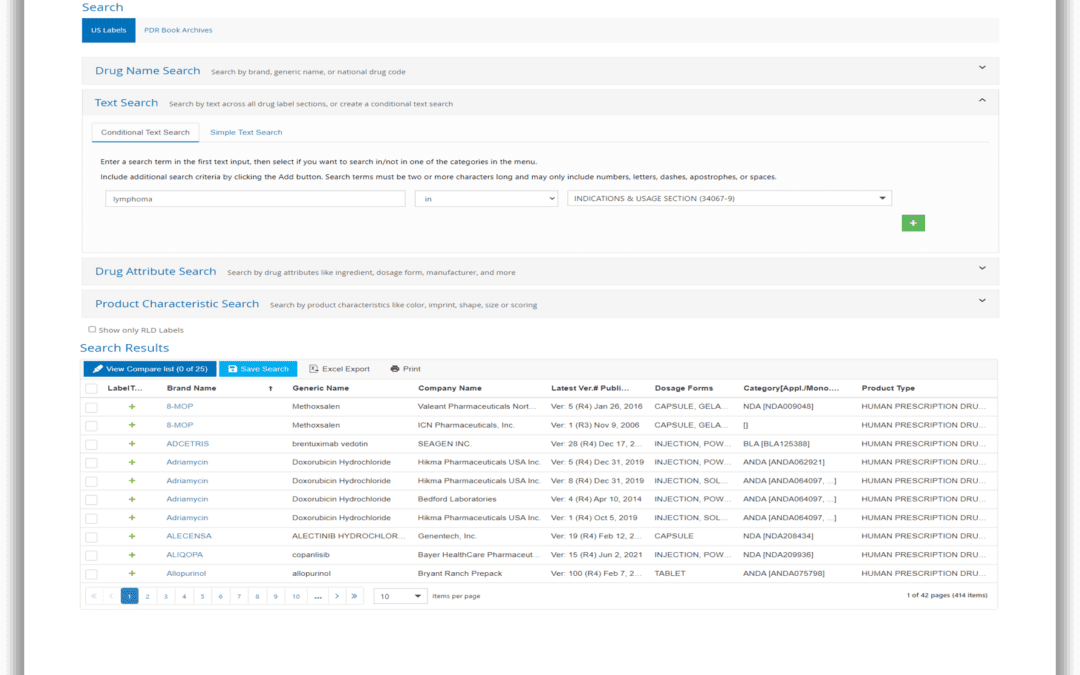
Greetings from Amsterdam! Earlier this month, Sales Development Representative Miriam Kniering represented Reed Tech Life Sciences at the RAPS Euro Convergence conference in Amsterdam. Upon Miriam’s return, we took some time to sit down and ask her a few questions...