All Blog Posts
Search or browse the Reed Tech Life Sciences blog posts archive below.Search All Blog Posts:
FDA Issued Final Guidance on Over-the-Counter Monograph Submissions in Electronic Format
The FDA has issued the final guidance “Providing Over-the-Counter Monograph Submissions in Electronic Format.” This new guidance mandates electronic submission of all Over-the-Counter (OTC) monograph materials, marking a significant shift in the regulatory landscape. By transitioning to electronic submissions, the FDA has expressed aim to streamline the review process, enhance transparency and facilitate faster decision-making for OTC drug approvals.
Takeaways from DIA Global Annual Meeting in San Diego
This past June, Reed Tech Life Sciences team members attended the 60th Anniversary of the DIA Global Annual Meeting in San Diego, California.
Reg Ops Radar: eCTD & Regulatory Updates
Industry is currently seeing rapid changes and improvements to existing eCTD formats, requirements and suggested uses from FDA. Here at LexisNexis Reed Tech, we want to be sure that our customers are not only up-to-date with, but understand, these new developments. To support this knowledge share, our in-house team of eCTD experts will be offering insights on a regular cadence this summer.
July 31st Deadline Approaching for CARES Act Report
The FDA CARES Act requires drug manufacturers, repackers and relabelers to submit annual reports on drug production volumes. Here are the key points: Deadline: July 31, 2024, for the 2023 calendar year; Who Must Report: All FDA-registered facilities under section 510(j)(3) of the Federal Food, Drug & Cosmetic Act; What to Report: Annual production volumes for each listed drug.
EUDAMED-Now or Later?
With the EUDAMED go-live date rescheduled multiple times, manufacturers face conflicting strategies of when to make UDI/Device registration submissions to EUDAMED.
Shaping the Future of Biopharma: Key FDA Decisions & Their Impact on Industry Dynamics
The biopharma industry is poised for significant transformation in the coming years, with several critical FDA decisions expected in the near future. These decisions will not only impact individual companies and products but have far-reaching implications for the entire industry— shaping market dynamics, investment strategies and the trajectory of innovation. As industry leaders navigate this complex landscape, they must remain vigilant of FDA’s actions and adapt their strategies to capitalize on emerging opportunities while mitigating potential risks.
LexisNexis® Reed Tech expands MedTech Regulatory Compliance Solutions and Services for medical device companies
LexisNexis® Reed Tech, a leading provider of regulatory submissions, data management, and analytics solutions for the life sciences industry, is expanding its services portfolio to a varied suite of regulatory compliance solutions and services to support medical device manufacturers, distributors, and related operations.
Market Research in Early-Stage Drug Concept and Discovery
During early-stage drug concept and discovery, Pharmaceutical companies will find it necessary to research the market for existing drug products for any number of reasons. These reasons can vary greatly depending on factors such as if the drug is Rx, OTC or biologic...
Benefits of a Singular Global UDI Vendor
As we continue to discuss optimal solutions to support the growth and compliance needs of industry, LexisNexis Reed Tech is committed to providing you with a single UDI vendor and platform. As such, here is a detailed analysis that highlights the tangible benefits of opting for a single, global end-to-end solution platform, compared to managing multiple vendor platforms.
Takeaways from 2024 IBA Cosmetics Convergence
LexisNexis Reed Tech was pleased to sponsor, present, and attend the recent IBA (International Beauty Association) Cosmetics Convergence Spring Symposium. In addition to having great conversations with our industry peers, we attended many educational sessions, especially those concerning the new MoCRA regulations, and are happy to share some highlights here. For more information about MoCRA requirements and deadlines, please contact [email protected]. To learn more about IBA and their upcoming events, please visit their website.
Expert Insights on the EU Proposed Legislation EUDAMED Rollout-Approved
On January 23, 2024, the European Commission proposed a legislative amendment to address two major issues in the EU Medical Device Regulation (MDR) and the In Vitro Diagnostics Regulation (IVDR).
FDA Plans for Artificial Intelligence and Medical Products
AI has the potential to revolutionize healthcare, and the FDA wants to ensure that patient safety remains the top priority while fostering these cutting-edge advancements. That’s where their four areas of focus come in – collaboration, regulatory clarity, standards and best practices and research.
MoCRA’s Impact on Private Label Cosmetic Manufacturers: Navigating the New Era of Beauty Compliance
The Modernization of Cosmetics Regulation Act (MoCRA) is here, and it’s about to shake up the industry. As the FDA gears up to implement MoCRA by July 2024, it’s time for private label manufacturers to get their ducks in a row and embrace the new era of beauty compliance.
FDA Releases Drug Amount Report Final Guidance and Updated Deadlines
After an 829 day wait from the initial draft guidance, the FDA has issued the long-awaited Final Guidance on Reporting Amount of Listed Drugs and Biological Products Under Section 510(j)(3) of the Federal Food, Drug, and Cosmetic Act – or as Reed Tech has taken to...
EC Proposal Updates on IVDR Transition & EUDAMED Rollout
Quick Insights from our experts on the EC Proposal concerning the IVDR transition and proposed MDR/IVDR amendment, possibly affecting the EUDAMED rollout. These are short segments created from our latest presentation, prepared exclusively for UDI customers.
EUDAMED UDI Regulations: Best Practices for the New Year
Start the year with clean data and a firm understanding of what you must do for EUDAMED UDI compliance in 2024. UDI compliance readiness can be confusing. To help, we’ve gathered top tips from expert Gary Saner regarding upcoming milestones in the EUDAMED roadmap.
2024 MoCRA Update: Delays, Enforcement and More
As the first US cosmetic regulation in over 80 years is finally being implemented, Reed Tech is keep a close eye on all evolving mandates, requirements and deadlines to communicate all relevant information to our customers. As such, Reed Tech subject-matter experts,...
Reed Tech Submits First M2M UDI Submission to the AusUDID Pre-Production System
In recent news, Reed Tech became the first company to submit a device record (machine-to-machine) to the AusUDID Pre-Production system successfully.
Understanding Basic UDI-DI in EUDAMED
By submitting product data to health authority databases, the medical device industry can help ensure the safety and traceability of devices. One crucial element is the European Commission/EUDAMED-specific concept of Basic UDI-DI.
MoCRA Submission Encouraged by Dec. 29, 2023; Enforcement Delayed
On November 8, 2023, the Food and Drug Administration (FDA) announced that it will be ready to accept registration and listing information for the Modernization of Cosmetics Regulation Act of 2022 (MoCRA) by the statutory deadline of December 29, 2023 and encourages companies to meet that deadline.
Update on EUDAMED Timeline
The EUDAMED timeline has experienced several delays and revisions since its inception, primarily due to the complexity and magnitude of the project.
Navigating the AusUDID Rollout: A New Era for Medical Device Identification
Explore the significance of the AusUDID rollout, its objectives, and how the TGA requirements for UDI differ from FDA regulations.
Health Canada Releases XML PM Draft Guidance
Following a lengthy period of anticipation, Health Canada has released Draft Guidance Document Preparation of the Product Monograph in Extensible Markup Language (XML) Format and sample files.
Revisions to FDA-Forms 365h and 1571
The US Food and Drug Administration (FDA) has recently made updates to forms 365h and 1571. Form 365h is “Application to Market a New or Abbreviated New Drug or Biologic for Human Use”. These applications are commonly referred to as NDA, ANDA or BLA. Form 1571 is “Investigational New Drug Application (IND)”. These are among the most important eCTD forms involved in your drug or biologic application process.
FDA Releases MoCRA Draft Guidance
On August 7, 2023, the Food and Drug Administration (FDA) released draft guidance providing recommendations and instructions pertaining to the collection and submission of information related to the Modernization of Cosmetics Regulation Act of 2022 (MoCRA), specifically, to assist persons submitting cosmetic product facility registrations and product listings to FDA.
Embracing EUDAMED UDI Compliance Timing: Why Starting the Process Now is Crucial for Medical Device Manufacturers
EUDAMED UDI Compliance is a critical aspect of the European Medical Device Regulation (MDR) that requires manufacturers to adopt a standardized UDI system for better traceability and safety of medical devices within the EU market. The compliance process may seem...
Understanding FDA Exemptions to Unique Device Identification (UDI) Requirements
The Food and Drug Administration (FDA) has implemented the Unique Device Identification (UDI) system to enhance patient safety, improve post-market surveillance, and facilitate the identification of medical devices. The UDI system requires manufacturers to assign a...
Understanding FDA UDI Compliance Requirements for Medical Device Manufacturers
What are the potential consequences for medical device manufacturers not following through with product data submissions and labeling requirements? In a recent inspection, violations were identified regarding a manufacturer’s Unique Device Identification (UDI)...
FDA has Updated eCTD Guidance to Recommend Structure-Data Files
Effective March 10, 2023, Health Canada changed submission requirements for second-language product monographs for human drugs. This change no longer requires the second language product monograph at the time of submission filing or review. However, both English and French languages need to be on the Drug Product Database (DPD) online and Drug and Health Product Portal (DHPP).
LexisNexis® Reed Tech expands portfolio to offer Electronic Common Technical Document Submission Publishing Services
LexisNexis® Reed Tech, a leading provider of regulatory submissions, data management, and analytics solutions for the life sciences industry, is expanding its services portfolio to offer end-to-end eCTD solutions and services to support manufacturers, distributors,...
WHO Responds to Global Rx Dependency Crisis
Over the years, Global Health Authorities have begun to work towards regulatory harmonization. Of course, the World Health Organization (WHO), a special agency of the United Nations responsible for international public health and established in 1948, is often at the...
Med Devices Missing in GUDID and Obsolete GMDN Codes in GUDID
Courtesy notification of news from FDA: The U.S. Food and Drug Administration (FDA) recently initiated two initiatives to improve the completeness and quality of the Global Unique Device Identification Database (GUDID). As a medical device manufacturer or other...
Reed Tech Takeaways from RAPS Euro Convergence
Greetings from Amsterdam! Earlier this month, Sales Development Representative Miriam Kniering represented Reed Tech Life Sciences at the RAPS Euro Convergence conference in Amsterdam. Upon Miriam’s return, we took some time to sit down and ask her a few questions...
LexisNexis Reed Tech teams up with RegDesk, a leading regulatory information management platform
Horsham, Pa., USA – May 25, 2023The collaboration of the leading providers of Unique Device Identification (UDI) for global health authorities and RIMS compliance platform, supports global medical device companies. LexisNexis Legal & Professional today announced,...
Health Canada Now Requiring Second-Language at Post-Authorization Phase
Effective March 10, 2023, Health Canada changed submission requirements for second-language product monographs for human drugs. This change no longer requires the second language product monograph at the time of submission filing or review. However, both English and French languages need to be on the Drug Product Database (DPD) online and Drug and Health Product Portal (DHPP).
Coffee Talk with Reed Tech – EU EUDAMED Testing Update
In the latest edition of 'Coffee Talk', Reed Tech UDI experts, Gary Saner (Sr. Manager of Information Solutions) and John Lorenc (Director Product Management, Medical Devices), discuss EU EUDAMED access connection issues, who has been affected, what scenarios had...
Simplifying Annual Drug Sample Reporting
Pharma manufacturers and authorized distributors need to submit distribution reports electronically in an eXtensible Markup Language (XML) format to comply with the guidance entitled Reporting Drug Sample Information Under Section 6004 of the Affordable Care Act. This guidance fulfills the Drug Sample Transparency Act specification of ACA Section 6004. These amounts must be reported each year by April 1st for the previous calendar year.
LexisNexis® Reed Tech™ and ONIX Life Sciences Expand Strategic Alliance to Guide Pharma Customer to Market Faster
HORSHAM, Pa., February 14, 2023 /PRNewswire/ LexisNexis Reed Tech has teamed up with ONIX, a boutique regulatory affairs and operations consultancy, supporting global eCTD submissions. Professionals in the life sciences industry rely on unique regulatory affairs...
MoCRA: The Future of Cosmetic Regulations
The Modernization of Cosmetics Regulation Act of 2022 (MoCRA) was signed into law on December 29, 2022. This law, which provides a major overhaul of existing cosmetic regulations, requires the Food and Drug Administration (FDA) to create Good Manufacturing Practices for all cosmetics manufacturers. The law states that these established practices must include mandatory reporting of serious adverse health events caused by cosmetic products and mandatory testing of asbestos levels. This law will also include updates to cosmetic listing requirements.
Missed FDA Annual Deadlines? It’s not too late to keep your drug active!
The Food and Drug Administration (FDA) requires drug manufacturers to keep their listings updated each year. Most of the deadlines fall on December 31st. What happens if a manufacturer misses these deadlines? In most cases, the drug is in danger of becoming...
Medical Device Regulatory Requirements – Japanese Guidelines
This is a quick summary of the December 14, 2022 Emergo Webinar presented by Kenji Yashiro on the topic: Medical Device Regulatory Requirements-Japanese Guidelines Key Takeaways: This blog will cover just a few high-level takeaways from the presentation by Kenji...
Reed Tech Life Sciences Team Gives Back: An inside look at volunteering with Cradles to Crayons
Giving Back: Last week the Reed Tech Life Sciences team spent some time out of the office to give back to the Philadelphia community. The team met up at Cradles to Crayons, a non-profit serving children in need from birth through age 12 with the everyday essentials...
New FDA Requirement: New and Updated REMS Files to be Submitted in SPL Format
As of December 28, 2022, all new REMS (Risk Evaluation and Mitigation Strategy) submissions and all REMS updates will be required to be submitted in SPL (Structured Product Labeling) format per the FDA. This is in compliance with the Final Guidance on REMS issued by...
Class I UDI Inventory Exception: What You Need to Know about FDA Compliance
With the US Class I UDI enforcement quickly approaching, you may be wondering “what about older inventory”? In 2013, the FDA granted a UDI exception for 3 years after the corresponding Compliance Date for finished medical devices manufactured and labeled without...
Vision Expo West and Eyewear Industry UDI
Reed Tech Life Sciences representative Patti Shragher attended The Vision Expo West 2022 conference, on September 14th – 17th. This year's event took place at the Venetian Convention Center & Expo in Las Vegas, Nevada. We asked a few questions about her...
Combination Products: Regulatory Requirements and How To Comply
Combination Products continue to be at the forefront of regulatory chatter. Our customers, both medical device and pharmaceutical, are regularly coming to us with questions surrounding this topic. How do I decide if my product is a combination product? What are the...
Status Update for Australia TGA UDI
The Australia Therapeutic Goods Administration (TGA) has announced several updates to the go-forward plan for implementing UDI compliance News of note: • TGA has announced that they will have a similar model to the FDA, adopting some elements of the EUDAMED model• A...
FDA Class I Medical Device UDI Due December 2022
Update on FDA Class I UDI as of July 22, 2022 Final guidance has been issued by the US Food and Drug Administration (FDA) for labelers of Class I medical devices. In an effort to help the industry remain focused on high-quality Unique Device Identification (UDI)...
HL7 Structured Product Labeling and the True Cost of the Free FDA Web Interface
Did you know? The FDA Class I deadline enforcement is occurring on December 8, 2022. Many in the medical device industry make plans to use the free FDA Web Interface based on the assumption that they don’t have enough products to warrant the use of Structured Product...
What’s Been Happening with Health Canada XML PM Mandates?
Reed Tech recently presented a webinar to update customers with the most recent information about XML product monograph (XML PM) and how to best navigate them. In the webinar, Gary Saner, Sr. Manager, Information Sciences, discusses the history of Health Canada...
Preparing, Managing and Monitoring Medical Device UDI
In today’s ‘Coffee Talk’ we are chatting with Linda Morehouse, Account Executive with Reed Tech Life Sciences. Linda specializes in helping medical device manufacturers with Unique Device Identification solutions for global health authorities like US FDA, EUDAMED and...
DIA Global Labeling Takeaways: Patient Materials and Health Literacy
Reed Tech habitually attends industry conferences and forums to share our expertise and gain knowledge from our industry colleagues and friends. This past month, we attended DIA’s virtual Global Labeling conference. Some of the main topics addressed in this year’s...
LexisNexis® Reed Tech™ and Schlafender Hase® Alliance Provides Mutual Customer Benefits for Pharmaceutical and Medical Device Companies
HORSHAM, Pa., April 26, 2022 /PRNewswire/ -- Reed Technology and Information Services Inc. (Reed Tech™), a leading provider of data management and analytics solutions for the life sciences industry, today announced an alliance with Schlafender Hase®, the market...
FDA Drug Distribution Amounts Report: Frequently Asked Questions and More
In late 2021, the US Food and Drug Administration (FDA) is instited a new annual distribution report, Reporting Amount of Listed Drugs and Biological Products Under Section 510(j)(3) of the Federal Food, Drug, and Cosmetic Act, for all listed drugs. According to...
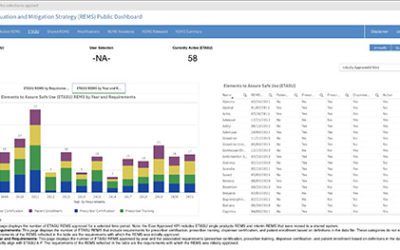
Public Dashboard Now Available for REMS Data
A new public dashboard has been launched by the Food and Drug Administration (FDA) for public access to data for drugs with approved Risk Evaluation and Mitigation Strategy (REMS.) According to FDA, This new dashboard includes visualizations and charts for total and...
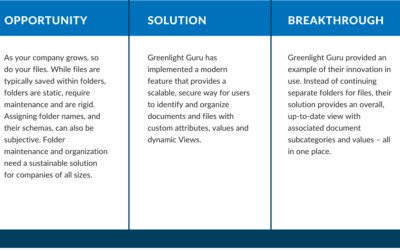
Organizing and Finding Documents Easily for Medical Device Companies, Featuring Greenlight Guru
At Reed Tech Life Sciences, we know the challenges of locating and securing important documentation for both internal and external downstream users. In a recent webinar presentation, Reed Tech Alliance member, Greenlight Guru, showcased how their solution empowers...
How Can You Prepare for Unique Device Identification Product Data Submission in Asia?
Reed Tech subject matter experts, Gary Saner, Information Solutions Senior Manager and Patti Shragher, Medical Device Senior Account Executive and Team Lead, host a webinar discussion dedicated to Unique Device Identification (UDI) requirements for health authorities...
LexisNexis® Reed Tech and Greenlight Guru Announce Strategic Alliance to Guide Customers to Market Faster
LexisNexis® Reed Tech has teamed up with Greenlight Guru, the leading Medical Device Success Platform for medical device companies. Horsham, Pa., USA – February 24, 2022 – In response to the needs expressed by medical device manufacturers for ways to effectively...
How Does Software as a Medical Device Relate to Unique Device Identification?
Determining Software as a Medical DeviceIt is critical to first determine if the software article is a Device Component, a Device Accessory, or a Standalone Device. A Device Component is any raw material, substance, piece, part, software, firmware, labeling, or...
Is My Product a Medical Device?
A frequent question on medical devices concerns determining ‘if’ a product is defined by US FDA as a ‘medical device’. Intended Use and Indications for Use are key determiners and FDA provides clear guidance. Packaging and accessory definitions can be reasons for...
Updated Deadline: FDA Announces New Annual Drug Distribution Reporting Requirement
The Food and Drug Administration (FDA) has informed industry that the February 15, 2022 date for submitting 2020 data per Reporting Amount of Listed Drugs and Biological Products Under Section 510(j)(3) of the Federal Food, Drug, and Cosmetic Act, originally referred...
What is a UDI label and UDI requirements?
What is UDI?Unique Device Identification is a globally unique, unambiguous identification comprised of a Device Identifier (DI) and a Production Identifier (PI). UDI is specific to a device model and version of that device on the market.How Do You Create the UDI?The...
FDA Withdraws 216 ANDAs
Abbreviated new drug applications (ANDAs) are subject to complying with requirements of FDA annual reporting—often the Q4 submission deadlines including blanket no-change certification (BNCC) or establishment registration (ER). Due to non-compliance with these annual...
Overview of Pharma Annual Deadlines and Requirements
Each year pharmaceutical companies, including both manufacturers and private label distributors, are subject to numerous guidances and mandates to develop and maintain compliance with the FDA. Though every company is different and each situation is unique, the...
Proposed Changes to OTC Sunscreen GRASE and Labeling Regulations
The FDA has proposed new changes to the regulations regarding over-the-counter sunscreen products. Perhaps the most impactful change to manufacturers, the FDA seeks to significantly decrease the ingredients that are generally recognized as safe and effective (GRASE)...
Medical Device Classifications in Global Markets and Health Authorities
When marketing medical devices around the globe, manufacturers face the significant challenge of meeting requirements of multiple regulatory agencies.An important component of achieving regulatory approval is a classification of the medical device, according to the...
FDA GUDID Class I Reminder
(This blog was updated July 22, 2022) What is the Global Unique Device Identification Database, also known as GUDID? It is a database that contains key device information submitted to the FDA, including Unique Device Identifier (UDI) product data. Within the FDA...
EU’s Requirement for Implant Cards Provides Key Information for Patients
The European Union (EU) requires manufacturers to supply ‘implant cards’ for patients with implanted medical devices, as prescribed in Article 18 of Regulation (EU) 2017/745 on medical devices. The requirement’s purpose is to give patients easy access to important...
New Drug Application (NDA): Back to the Basics
What is a New Drug Application (NDA)?Every new drug in the United States needs to come to market through a New Drug Application with the FDA. According to the FDA, “The NDA application is the vehicle through which drug sponsors formally propose that the FDA approve a...
LexisNexis Reed Tech Life Sciences receives ISO/IEC 27001:2013 certification for international information security standard
ISO 27001 is the leading international standard for information security management systems (ISMS) and thus the most important cyber security certification. It defines the requirements for the introduction, implementation, monitoring and improvement of an information...
Customer Q&A: OMUFA Update
Recently, Reed Tech has been receiving many questions from our Pharma customers regarding the Over-The-Counter Monograph User Fee Program. As a response, we enlisted our colleague and friend, Carolina Wirth, Of Counsel, Arnall Golden Gregory LLP to discuss these...
Updated Survey Shows Most Are Continuing Preparation for EU EUDAMED
Polls conducted May 20, 2021 Survey ResultsAre you as a medical device manufacturer, in the middle of preparing for the EU Medical Device Regulation (MDR)? Many are trying to figure out their response to the European Commission's notice that the EUDAMED launch was...
China NMPA UDI and Device Registration Basics
In China, key regulatory policies originate with the State Council (Executive Branch). At the policy level, regulations for medical devices started in 2016 with a five-year plan. For regulatory compliance, typically there is a registration process, clinical evaluation...
LexisNexis Reed Tech is proud to be recognized by the Dental Trade Alliance as a solution provider for Unique Device Identification (UDI) to U.S. FDA and other Global Health Authorities
HORSHAM, Pa., May 18, 2021 /PRNewswire/ -- Reed Technology and Information Services Inc. (Reed Tech™), a leading provider of data management and analytics solutions for the life sciences industry, is proud to be recognized by the Dental Trade Alliance (DTA),...
What to Know About NDC: The Basics of National Drug Codes
Per the Drug Listing Act of 1972, part of the Food and Drug Administration (FDA) requirement to electronically list drug products (Rx and OTC) includes the assignment of a National Drug Code (NDC). Drug establishments are required to provide the FDA with a current...
Why You Need a UDI Specialist
Did you know there are multiple health authorities around the globe with current or future requirements for medical device product data specifically for Unique Device Identification (UDI) standards? The list continues to grow (US FDA, EU EUDAMED, South Korea MFDS,...
How to Utilize an Authorized Representative in UDI Submissions to Health Regulators
For medical device manufacturers and distributors, UDI product data submission to Health Authorities/Regulators is a multi-step process with rules and goals, much like a ‘team sport’.My local affiliate (Authorized Representative) handles UDI in that region, how can we...
Unique Device Identification (UDI) Update for South Korea
Are UDI requirements in South Korea similar to other health regulators? The short answer is 'not exactly'. In this episode of Reed Tech Insights, we discuss the legislation, policy, timing and data attributes involved in the South Korean Unique Device Identification...
OTC Monograph Fees Announced… Not so fast!
Co-Authored by David Wilson, Sr. Account Executive, Reed Tech, and Carolina Wirth, Of Counsel, Arnall Golden Gregory, LLP On December 29, 2020, the U.S. Food and Drug Administration published a Federal Register notice setting the fee rates under the Over-the-Counter...
Evolution of UDI: A Look Back at RAPS 2020 Euro Convergence
Reed Tech attended RAPS 2020 Euro Convergence – this year live and online. We were able to connect virtually and share ideas with our colleagues, customers and leading industry experts at our booth, during networking hours and through live sessions. It was great to...
Ensure Imports are Not Detained Due to FDA Non-Compliance
In the final webinar of 2020, Reed Tech invited an expert attorney to discuss import compliance and how it ties into FDA compliance. David Wilson, Reed Tech, and Jennifer Diaz, Esq., Diaz Trade Law, discussed many topics ranging from compliant importation and FDA...
UDI Assignment for Spectacle Lenses & Readers in EUDAMED
Are makers of eyewear products subject to Unique Device Identification (UDI) requirements for compliance with EUDAMED? Recent news would indicate the answer is ‘yes’. For those manufacturers of eyewear lenses and frames, a recently posted guidance document from the EU...
When is UDI required for drug-device combination products?
From the FDA point of view, there are some very nuanced rules about how UDI is applied to combination products. When defining a combination product, 21 CFR 3.2 explains several scenarios. As a ‘single-entity’ combination, two or more regulated components...
5 Key Takeaways: What You Need to Know – OTC Drug Reform & the CARES Act (Monographs & OMUFA)
For a thorough briefing on the latest with OTC Drug Reform and the CARES Act, we had a conversation with Carolina Wirth, Of Counsel, at Arnall Golden Gregory, LLP. The presentation was hosted by Gary Saner, Senior Manager, Information Solutions, Reed Tech and recorded...
Saudi Arabia (SFDA) Unique Device Identification Update for Med Device Manufacturers
Background Saudi Food and Drug Authority (SFDA) issued final “Guidance on Requirements for Unique Device Identification (UDI) for Medical Devices” (MDS-G34) (MDS-G34 at HIBCC) in April 2019. The guidance published an update in December 2019 and a third update released...
Reed Tech SingleSource™ for Drug Products – New Enhancements
SingleSource™ for Drug Products helps regulatory professionals manage drug product meta-data with an intuitive interface, filters, and prompts. With new enhancements soon to be released, SingleSource™ for Drug Products is even more intuitive and...
The Anatomy of a National Drug Code (NDC)
Per the Drug Listing Act of 1972, part of the Food and Drug Administration (FDA) requirement to electronically list drug products (Rx and OTC) includes the assignment of a National Drug Code (NDC). An NDC is a 10 digit number that uniquely and universally identifies a...
Electronic Drug Listing and Registration Guides
To learn the main requirements for Electronic Drug Registration and Labeling (eDRL), download a quick-reference guide with step-by-step instructions and ways to simplify and expedite the process. Download your free guide here. The eDRL How-to Guide answers questions...
UDI Labeling (Unique Device Identification): Best Practices
Unique Device Identification (UDI) labeling remains a hot topic among medical device manufacturers under pressure to comply with UDI requirements for US FDA GUDID and other emerging health authorities around the globe. EU EUDAMED is underway and China's NMPA, South...
Navigator for Drug Labels: Faster product label creation means faster time to market for rare-disease drug manufacturer
A front-line perspective on the value of Reed Tech Navigator™ for Drug Labels for a rare-disease biopharmaceutical manufacturer. In working with life sciences customers, we often hear recurring themes on product labeling. Some companies lack the time or...
UDI and 21 CFR Part 11
Medical device manufacturers working to comply with the FDA’s Unique Device Identification mandate have more than one set of regulations to keep in mind. Fortunately, some of the regulations have been in place for some time and will be at least somewhat familiar to...
Fact vs Fiction: UDI in China and Global Data Pools
Introduction The National Medical Products Administration (NMPA) is the regulating body in China for drugs and medical devices. The NMPA is the authoritative group that drafts laws and regulations for drugs, medical devices, and cosmetics. They also establish medical...
Global Data Synchronization Network
Why your product data needs to be in GDSN…NOW! If you manufacture medical devices, you know the challenges in managing accurate, current, and complete product data and sharing it with your trading partners. Your data consumers, both internal and external, trust your...
Fact vs Fiction: GDSN Connection
Author: John Lorenc, Sr. Manager Regulatory Solutions, Reed Tech Fiction: Most global health authorities subscribe to a GDSN data pool, therefore, UDI compliance can be achieved by a standard GDSN data pool publication. Fact: One of the largest health authorities,...
Reed Tech Selected by The Vision Council as the full-service solution for UDI
Reed Tech is proud to be recognized as the full-service solution for Unique Device Identification (UDI) to US FDA and other Global Regulators by The Vision CouncilHORSHAM, PA. (PRWEB) JANUARY 07, 2020 Reed Technology and Information Services Inc. (Reed Tech™),...
Reed Tech Insights: US FDA and EU EUDAMED Comparisons
Are data elements for EU EUDAMED similar to US FDA for unique device identification (UDI) submission? The short answer is, 'not exactly.' In this series of Reed Tech Insights, we take a deep-dive into the UDI data attributes of US and EU in specific areas, noting the...
A Closer Look at the FDA’s UDI Guidance: Which Convenience Kits Need UDI? Which Don’t?
Pop quiz: For the purposes of FDA Unique Device Identification (UDI), is an anterior cruciate ligament (ACL) procedure kit considered one medical device, i.e., a “convenience kit?” That question is one of many addressed by the FDA’s final guidance on Unique Device...
Webinar Recording: Challenges and Considerations for Building Your Own UDI Solution
Is your team evaluating UDI regulatory submissions for EU and other regions? All the complexity around UDI regulatory (and commercial) requirements across the globe create serious challenges for product data management. When considering what’s at stake, a...
Meet the Team at Reed Tech – Patti Shragher
Patti Shragher Account Executive, Reed Tech Life SciencesPatti enjoys solving problems with technology and takes pride in strategic challenges. She consults with customers and prospects all over the globe on Reed Tech solutions for regulatory compliance, product data...
Meet the Team at Reed Tech – Andrew Pfeifer
Andrew Pfeifer Senior Account Executive, Reed Tech Life SciencesAndrew is an experienced guiding force for medical device professionals, having spent the last 5 years in UDI related applications. He specializes in understanding the complexities of product data...
UDI Implementation Tips and GDSN Data Management
Tracking and Tracing Medical Devices: 4 Ways to Simplify Your UDI and GDSN Data Management By: John Lorenc, Senior Manager Regulatory Solutions — Life Sciences Reed Tech Tracking medical devices throughout the healthcare supply chain and delivery system has proven...
Reference Checklist: FDA Medical Device UDI Regulation Records, Reports, SOPs
The FDA Unique Device Identification (UDI) regulation requires manufacturers to identify their medical devices with a UDI placed on their product and package labels. Of equal importance, the FDA UDI regulation also requires manufactures to report medical device...
Blog: NHS England Extends eProcurement Deadline for In Vitro Diagnostic Devices: Q&A with Gary Saner
In December 2018, the National Health Service (NHS) England announced an extension to its eProcurement deadline for in-vitro diagnostic devices. Instead of being required to comply by September 2019, manufacturers now have until September 2021. I caught up with Gary...
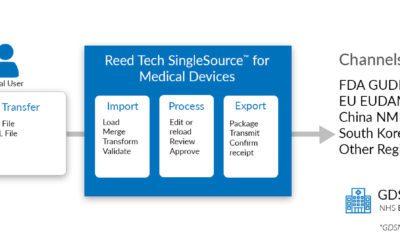
Reed Tech® Introduces Reed Tech SingleSource™ for Medical Devices Supporting UDI Requirements around the Globe
HORSHAM, Pa., March 25, 2019 /PRNewswire/ -- Reed Technology and Information Services Inc. (Reed Tech), a leader in data management and analytic services and solutions for the Life Sciences industry, announces the launch of Reed Tech SingleSource™ for Medical...
Combination Product: What to Consider for both Medical Device and Drug Constituents
What are the required actions when a product includes both a medical device constituent and a drug constituent? Learn more from Reed Tech
What is a GLN (Global Location Number)?
Global Location Number (GLN) A Global Location Number (GLN) is a 13 digit number that acts as a key for identifying the location, whether physical or digital, and the function or entity of a company across the supply chain. A GLN gives companies complete flexibility...
Use GDSN to Increase Your Corporate Value
What is the Global Data Synchronization Network? The GDSN is the largest online product data network. It’s a secure network that allows any agents, such as manufacturers, retailers, distributors, and wholesalers to share validated product information. Because of its...
Reed Tech® Introduces Reed Tech SingleSource™ for Medical Devices, Advancing the Ability to Manage and Share Device Product Data
Reed Tech, a LexisNexis® company and leading provider of data management and analytics solutions for the life sciences industry, introduces Reed Tech SingleSource for Medical Devices, a data management tool built specifically to meet the needs of medical device...
UK NHS eProcurement: The What, Who, Why and When
If your organization markets medical devices in the United Kingdom, you may have heard about the National Health Service (NHS) eProcurement program. Sometimes loosely referred to as the “U.K. UDI,” eProcurement shares some traits with FDA’s UDI program, but is quite...
GS1 UDI – How GDSN, GTIN, & GS1 Affect UDI
If you are involved in your organization’s compliance program for Unique Device Identification (UDI), you have probably heard a lot of “G terms” being thrown around, such as “GS1,” “GTIN” and “GDSN.” But what do these terms mean and where do they fit in with UDI...
UDI Data Security: Why it should be important to you
Data security has three facets—confidentiality, integrity and availability. Device labelers need to keep each of these areas in mind when it comes to managing the UDI data for their device portfolio. Confidentiality: Not all data you submit to FDA as part of a UDI...
A Risk-Based Approach to UDI Compliance
As medical device manufacturers scramble to meet Unique Device Identification (UDI) deadlines, a risk-based approach to UDI compliance will help ensure continuity within the quality system, consistency in UDI practice and application, accuracy of the associated data...
UDI: How do I assign Device Identifiers?
As you work on implementing UDI, you may be wondering how to assign the Device Identifier (DI) portion of the UDI to your products and how to make sense of the different levels of DIs in a single GUDID record. These are questions we receive from industry members all...
FDA GUDID UDI compliance: Accuracy required
Unique Device Identification (UDI) product data submissions require not just that your system for sending data to the FDA’s Global Unique Device Identification Database (GUDID) be in place and functioning smoothly. It requires that the data you send through that...
Handling UDI Submissions with Software-as-a-Service (SaaS)
Some medical device manufacturers seeking to comply with the FDA’s final rule on Unique Device Identification may want to turn to outside firms for help throughout the process. Others may already have the expertise in-house to handle their UDI compliance and FDA GUDID...
GUDID Account Creation: Five Steps to Follow
When medical device manufacturers prepare to submit their device data to the FDA in compliance with Unique Device Identification requirements, one of the prerequisite steps for all manufacturers is to establish a Global Unique Device Identification Database account....
What Your CEO Needs to Know about UDI
Leaders of the regulatory and labeling departments of a medical device manufacturer likely know that complying with the FDA’s final rule on Unique Device Identification (UDI) takes time and planning. But convincing the executives in the C-suite to allot the necessary...
Step 1 in UDI Compliance: Assembling the Right Team
After working with a number of medical device companies to successfully submit device records to the FDA, one important piece of advice from the experts at Reed Tech is to start by gathering the right team members from the beginning. Initially, many in the medical...
4 Questions to Ask Before Choosing a UDI Issuing Agency
As Implantable, Life-Sustaining and Life Supporting (I/LS/LS) and Class II medical device labelers have complied with the FDA’s final rule on Unique Device Identification (UDI), one of the first major decisions they needed to make: choose a device identifier (DI)...
UDI Data Submission: Who is Responsible?
As part of the FDA’s Unique Device Identification (UDI) mandate, medical device labelers are required to submit a data record for the devices they market in the United States to the FDA’s Global Unique Device Identifier Database (GUDID). But who does the FDA consider...
One Step in FDA UDI Compliance: The Dun & Bradstreet DUNS Number
In preparing for compliance with the U.S. Food and Drug Administration's final rule requiring Unique Device Identifiers (UDIs) for medical devices distributed in the U.S., there are a number of steps that device manufacturers should be aware of before submitting data...